Nimodipine sandbox
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
BOXED WARNING
See full prescribing information for complete Boxed Warning.
DO NOT ADMINISTER NIMODIPINE INTRAVENOUSLY OR BY OTHER PARENTERAL ROUTES. DEATHS AND SERIOUS, LIFE THREATENING ADVERSE EVENTS HAVE OCCURRED WHEN THE CONTENTS OF NIMODIPINE CAPSULES HAVE BEEN INJECTED PARENTERALLY (See WARNINGS and DOSAGE AND ADMINISTRATION).
|
Overview
Nimodipine sandbox is a Calcium Channel Blocker that is FDA approved for the {{{indicationType}}} of improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Hypotension, Diarrhea, Nausea, Headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Indication (For both capsule and solution)
- Nimodipine is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I-V).
- Dosing information (In capsule)
- DO NOT ADMINISTER NIMODIPINE CAPSULES INTRAVENOUSLY OR BY OTHER PARENTERAL ROUTES (see WARNINGS). If Nimodipine is inadvertently administered intravenously, clinically significant hypotension may require cardiovascular support with pressor agents. Specific treatments for calcium channel blocker overdose should also be given promptly.
- Nimodipine is given orally in the form of soft gelatin 30 mg capsules for subarachnoid hemorrhage.
- Recommended dosage: 60 mg (two 30 mg capsules) PO q4h for 21 consecutive days.
- In general, the capsules should be swallowed whole with a little liquid, preferably not less than one hour before or two hours after meals. Grapefruit juice is to be avoided (See PRECAUTIONS, Drug Interactions). Oral nimodipine therapy should commence as soon as possible within 96 hours of the onset of subarachnoid hemorrhage.
- If the capsule cannot be swallowed, e.g., at the time of surgery, or if the patient is unconscious, a hole should be made in both ends of the capsule with an 18 gauge needle, and the contents of the capsule extracted into a syringe. A parenteral syringe can be used to extract the liquid inside the capsule, but the liquid should always be transferred to a syringe that cannot accept a needle and that is designed for administration orally or via a naso-gastric tube or PEG. To help minimize administration errors, it is recommended that the syringe used for administration be labeled “Not for IV Use”. The contents should then be emptied into the patient’s in situ naso-gastric tube and washed down the tube with 30 mL of normal saline (0.9%).
- Severely disturbed liver function, particularly liver cirrhosis, may result in an increased bioavailability of nimodipine due to a decreased first pass capacity and a reduced metabolic clearance. The reduction in blood pressure and other adverse effects may be more pronounced in these patients.
- Dosage should be reduced to one 30 mg capsule every 4 hours with close monitoring of blood pressure and heart rate; if necessary, discontinuation of the treatment should be considered.
- Strong inhibitors of CYP3A4 should not be administered concomitantly with nimodipine (See CONTRAINDICATIONS). Strong inducers of CYP3A4 should generally not be administered with nimodipine (See WARNINGS). Patients on moderate and weak inducers of CYP3A4 should be closely monitored for lack of effectiveness, and a nimodipine dose increase may be required. Patients on moderate and weak CYP3A4 inhibitors may require a nimodipine dose reduction in case of hypotension (See PRECAUTIONS, Drug Interactions)
- Dosing information (In solution)
- Administration Instructions
- Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. For all routes of administration, begin Nimodipine within 96 hours of the onset of SAH. Administer one hour before a meal or two hours after a meal for all routes of administration [see Clinical Pharmacology (12.3)].
- Administration by Oral Route
- Recommended oral dosage: 20 mL (60 mg) PO q4h for 21 consecutive days.
- Administration Via Nasogastric or Gastric Tube
- Using the supplied oral syringe labeled "ORAL USE ONLY", administer 20 mL (60 mg) every 4 hours into a nasogastric or gastric tube for 21 consecutive days. For each dose, refill the syringe with 20 mL of 0.9% saline solution and then flush any remaining contents from nasogastric or gastric tube into the stomach.
- Dosage Adjustments in Patients with Cirrhosis
- In patients with cirrhosis, reduce the dosage to 10 mL (30 mg) every 4 hours [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nimodipine sandbox in adult patients.
Non–Guideline-Supported Use
- Dosing information
Adjunct treatment of Dementia
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nimodipine sandbox in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nimodipine sandbox in pediatric patients.
Contraindications
The concomitant use of nimodipine with strong inhibitors of CYP3A4 such as some macrolide antibiotics (e.g., clarithromycin, telithromycin), some anti-HIV protease inhibitors (e.g., delaviridine, indinavir, nelfinavir, ritonavir, saquinavir), some azole antimycotics (e.g., ketoconazole, itraconazole, voriconazole) and some antidepressants (e.g., nefazadone) is contraindicated because of a risk of significant hypotension (See PRECAUTIONS, Drug Interactions)
Warnings
|
BOXED WARNING
See full prescribing information for complete Boxed Warning.
DO NOT ADMINISTER NIMODIPINE INTRAVENOUSLY OR BY OTHER PARENTERAL ROUTES. DEATHS AND SERIOUS, LIFE THREATENING ADVERSE EVENTS HAVE OCCURRED WHEN THE CONTENTS OF NIMODIPINE CAPSULES HAVE BEEN INJECTED PARENTERALLY (See WARNINGS and DOSAGE AND ADMINISTRATION).
|
Warning (capsule)
DEATH DUE TO INADVERTENT INTRAVENOUS ADMINISTRATION: DO NOT ADMINISTER NIMODIPINE INTRAVENOUSLY OR BY OTHER PARENTERAL ROUTES. DEATHS AND SERIOUS, LIFE THREATENING ADVERSE EVENTS, INCLUDING CARDIAC ARREST, CARDIOVASCULAR COLLAPSE, HYPOTENSION, AND BRADYCARDIA, HAVE OCCURRED WHEN THE CONTENTS OF NIMODIPINE CAPSULES HAVE BEEN INJECTED PARENTERALLY (SEE DOSAGE AND ADMINISTRATION). Reduced Efficacy with CYP3A4 Inducers: Concomitant use of strong CYP3A4 inducers (e.g. rifampin, phenobarbital, phenytoin, carbamazepine, St John’s wort) and nimodipine should generally be avoided, as nimodipine plasma concentration and efficacy may be very significantly reduced (see PRECAUTIONS, Drug Interactions). Moderate and weak inducers of CYP3A4 may also reduce the efficacy of nimodipine to a lesser extent. Patients on these should be closely monitored for lack of effectiveness, and a nimodipine dosage increase may be required. Moderate and weak CYP3A4 inhibitors include, for example: amprenavir, aprepitant, armodafinil, bosentan, efavirenz, etravirine, echinacea, modafinil, nafcillin, pioglitazone, prednisone and rufinamide.
PRECAUTIONS (capsule)
General
Blood Pressure: Nimodipine has the hemodynamic effects expected of a calcium channel blocker, although they are generally not marked. However, intravenous administration of the contents of nimodipine capsules has resulted in serious adverse consequences including death, cardiac arrest, cardiovascular collapse, hypotension, and bradycardia. In patients with subarachnoid hemorrhage given nimodipine in clinical studies, about 5% were reported to have had lowering of the blood pressure and about 1% left the study because of this (not all could be attributed to nimodipine). Nevertheless, blood pressure should be carefully monitored during treatment with nimodipine based on its known pharmacology and the known effects of calcium channel blockers. (see WARNINGS and DOSAGE AND ADMINISTRATION). Hepatic Disease: The metabolism of nimodipine is decreased in patients with impaired hepatic function. Such patients should have their blood pressure and pulse rate monitored closely and should be given a lower dose (see DOSAGE AND ADMINISTRATION). Intestinal pseudo-obstruction and ileus have been reported rarely in patients treated with nimodipine. A causal relationship has not been established. The condition has responded to conservative management.
WARNINGS AND PRECAUTIONS
Blood pressure should be carefully monitored during treatment with Nimodipine. In clinical studies of patients with subarachnoid hemorrhage, about 5% of nimodipine-treated patients compared to 1% of placebo-treated patients had hypotension and about 1% of nimodipine-treated patients left the study because of this. [see Adverse Reactions (6)].
Possible Increased Risk of Adverse Reactions in Patients with Cirrhosis
Given that the plasma levels of nimodipine are increased in patients with cirrhosis, these patients are at higher risk of adverse reactions. Therefore, monitor blood pressure and pulse rate closely and administer a lower dosage [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
Possible Increased Risk of Hypotension with Strong CYP3A4 Inhibitors
Concomitant use of strong inhibitors of CYP3A4, such as some macrolide antibiotics (e.g., clarithromycin, telithromycin), some HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, saquinavir), some HCV protease inhibitors (e.g., boceprevir, telaprevir), some azole antimycotics (e.g., ketoconazole, itraconazole, posaconazole, voriconazole), conivaptan, delaviridine, and nefazadone with nimodipine should generally be avoided because of a risk of significant hypotension [see Drug Interactions (7.2)].
Possible Reduced Efficacy with Strong CYP3A4 Inducers
Concomitant use of strong CYP3A4 inducers (e.g. carbamazepine, phenobarbital, phenytoin, rifampin, St John's wort) and nimodipine should generally be avoided, as nimodipine plasma concentration and efficacy may be significantly reduced [see Drug Interactions (7.3)].
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In clinical trials of nimodipine oral capsules in patients with SAH, eleven percent (92 of 823) of nimodipine-treated patients reported adverse events compared to six percent (29 of 479) of placebo-treated patients. The most common adverse event was decreased blood pressure in 4.4% of nimodipine-treated patients. The events reported with a frequency greater than 1% are displayed in Table 1 by dose.
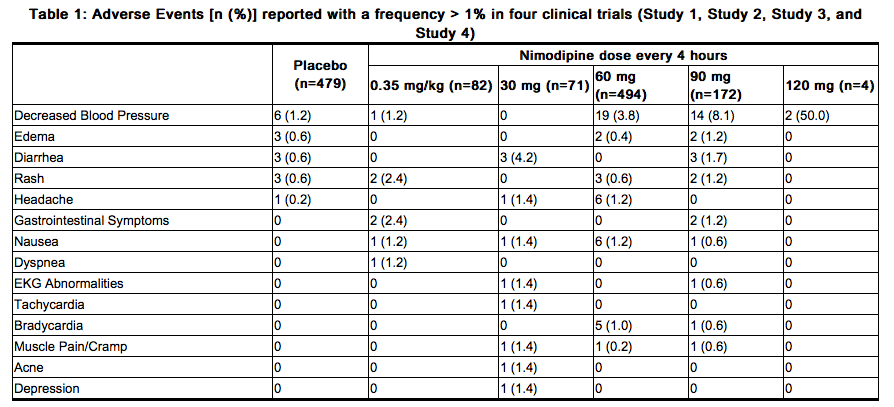
SAH is frequently accompanied by alterations in consciousness that may lead to an under-reporting of adverse experiences. As a calcium channel blocker, nimodipine may have the potential to exacerbate heart failure in susceptible patients or to interfere with A-V conduction, but these events were not observed in SAH trials.
Postmarketing Experience
FDA Package Insert for Nimodipine contains no information regarding Adverse Reactions.
Drug Interactions
Nimodipine is metabolized via the cytochrome P450 3A4 system located both in the intestinal mucosa and in the liver. Drugs that are known to either inhibit or to induce this enzyme system may therefore alter the first pass or the clearance of nimodipine. In addition, the blood pressure lowering effects of antihypertensives could be enhanced when taken concomitantly with nimodipine.
Inducers of CYP3A4
Nimodipine plasma concentration and efficacy may be significantly reduced when concomitantly administered with strong CYP3A4 inducers. Therefore strong CYP3A4 inducers (e.g. rifampin, carbamazepine, phenobarbital, phenytoin, St. John’s Wort) should generally not be administered concomitantly with nimodipine (see WARNINGS). Other moderate and weak inducers of CYP3A4 may also reduce the efficacy of nimodipine, although the magnitude of decrease in nimodipine plasma concentrations is not known. Patients on these should be closely monitored for lack of effectiveness, and a nimodipine dosage increase may be required. Moderate and weak CYP3A4 inducers include: amprenavir, aprepitant, armodafinil, bosentan, efavirenz, etravirine, Echinacea, modafinil, nafcillin, pioglitazone, prednisone and rufinamide.
Inhibitors of CYP3A4
Nimodipine plasma concentration can be significantly increased when concomitantly administered with strong inhibitors of the CYP3A4 system. As a consequence, the blood pressure lowering effect may be increased. Therefore strong CYP3A4 inhibitors should not be coadministered with nimodipine (See CONTRAINDICATIONS). Strong CYP3A4 inhibitors include some members of the following classes:
- macrolide antibiotics (e.g., clarithromycin, telithromycin,), - HIV protease inhibitors (e.g., delavirdine, indinavir, nelfinavir, ritonavir, saquinavir), - azole antimycotics (e.g., ketoconazole, itraconazole, voriconazole), - antidepressants (e.g. nefazodone) - grapefruit juice: after intake of grapefruit juice and nimodipine, the blood pressure lowering effect may last for at least 4 days after the last ingestion of grapefruit juice. Ingestion of grapefruit / grapefruit juice is therefore not recommended while taking nimodipine (See DOSAGE AND ADMINISTRATION). Nimodipine plasma concentration can also be increased in the presence of moderate and weak inhibitors of CYP3A4. If nimodipine is concomitantly administered with these drugs, blood pressure should be monitored, and a reduction of the nimodipine dose may be necessary. Moderate and weak CYP3A4 inhibitors include amprenavir, aprepitant, atazanavir, amiodarone, alprozalam, cyclosporine, cimetidine, erythromycin, fluconazole, fluoxetine, isoniazid, oral contraceptives, quinuprestin/dalforpristin, and valproic acid.
Blood pressure lowering drugs: Nimodipine may increase the blood pressure lowering effect of concomitantly administered anti-hypertensives, such as: – diuretics, – β-blockers, – ACE inhibitors, – A1-antagonists, – other calcium antagonists, – α-adrenergic blocking agents, – PDE5 inhibitors, – α-methyldopa. Blood pressure should be carefully monitored, and dose adjustment of the blood pressure lowering drug(s) may be necessary.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Pregnancy Category C. Nimodipine has been shown to have a teratogenic effect in Himalayan rabbits. Incidences of malformations and stunted fetuses were increased at oral doses of 1 and 10 mg/kg/day administered (by gavage) from day 6 through day 18 of pregnancy but not at 3 mg/kg/day in one of two identical rabbit studies. In the second study an increased incidence of stunted fetuses was seen at 1 mg/kg/day but not at higher doses. Nimodipine was embryotoxic, causing resorption and stunted growth of fetuses, in Long Evans rats at 100 mg/kg/day administered by gavage from day 6 through day 15 of pregnancy. In two other rat studies, doses of 30 mg/kg/day nimodipine administered by gavage from day 16 of gestation and continued until sacrifice (day 20 of pregnancy or day 21 post partum) were associated with higher incidences of skeletal variation, stunted fetuses and stillbirths but no malformations. There are no adequate and well controlled studies in pregnant women to directly assess the effect on human fetuses. Nimodipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nimodipine sandbox in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nimodipine sandbox during labor and delivery.
Nursing Mothers
Nimodipine and/or its metabolites have been shown to appear in rat milk at concentrations much higher than in maternal plasma. It is not known whether the drug is excreted in human milk. Because many drugs are excreted in human milk, nursing mothers are advised not to breast feed their babies when taking the drug.
Pediatric Use
Safety and effectiveness in children have not been established.
Geriatic Use
Clinical studies of nimodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dosing in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Nimodipine sandbox with respect to specific gender populations.
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
Oral
Monitoring
FDA Package Insert for Nimodipine contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
There have been no reports of overdosage from the oral administration of nimodipine. Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral vasodilation with marked systemic hypotension. Clinically significant hypotension due to nimodipine overdosage may require active cardiovascular support with pressor agents. Specific treatments for calcium channel blocker overdose should also be given promptly. Since nimodipine is highly protein-bound, dialysis is not likely to be of benefit.
Pharmacology
Mechanism of Action
Nimodipine is a calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells during depolarization as slow ionic transmembrane currents. Nimodipine inhibits calcium ion transfer into these cells and thus inhibits contractions of vascular smooth muscle. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood-brain barrier; concentrations of nimodipine as high as 12.5 ng/mL have been detected in the cerebrospinal fluid of nimodipine-treated subarachnoid hemorrhage (SAH) patients. The precise mechanism of action of nimodipine in humans is unknown. Although the clinical studies described below demonstrate a favorable effect of nimodipine on the severity of neurological deficits caused by cerebral vasospasm following SAH, there is no arteriographic evidence that the drug either prevents or relieves the spasm of these arteries. However, whether or not the arteriographic methodology utilized was adequate to detect a clinically meaningful effect, if any, on vasospasm is unknown.
Structure
Nimodipine belongs to the class of pharmacological agents known as calcium channel blockers. Nimodipine is isopropyl 2 -methoxyethyl 1,4 –dihydro -2,6 –dimethyl -4-(m-nitrophenyl) -3,5-pyridinedicarboxylate. It has a molecular weight of 418.5 and a molecular formula of C21H26N2O7. The structural formula is:
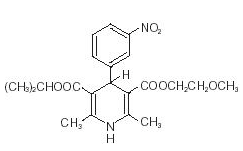
Nimodipine is a yellow crystalline substance, practically insoluble in water.
Pharmacodynamics
FDA Package Insert for Nimodipine contains no information regarding pharmacodynamics.
Pharmacokinetics
In man, nimodipine is rapidly absorbed after oral administration, and peak concentrations are generally attained within one hour. The terminal elimination half-life is approximately 8 to 9 hours but earlier elimination rates are much more rapid, equivalent to a half-life of 1-2 hours; a consequence is the need for frequent (every 4 hours) dosing. There were no signs of accumulation when nimodipine was given three times a day for seven days. Nimodipine is over 95% bound to plasma proteins. The binding was concentration independent over the range of 10 ng/mL to 10 µg/ml. Nimodipine is eliminated almost exclusively in the form of metabolites and less than 1% is recovered in the urine as unchanged drug. Numerous metabolites, all of which are either inactive or considerably less active than the parent compound, have been identified. The metabolism of nimodipine is mediated by CYP3A4. Because of a high first-pass metabolism, the bioavailability of nimodipine averages 13% after oral administration. The bioavailability is significantly increased in patients with hepatic cirrhosis, with Cmax approximately double that in normals which necessitates lowering the dose in this group of patients (see DOSAGE AND ADMINISTRATION). In a study of 24 healthy male volunteers, administration of nimodipine capsules following a standard breakfast resulted in a 68% lower peak plasma concentration and 38% lower bioavailability relative to dosing under fasted conditions. In a single parallel-group study involving 24 elderly subjects (aged 59-79) and 24 younger subjects (aged 22-40), the observed AUC and Cmax of nimodipine was approximately 2-fold higher in the elderly population compared to the younger study subjects following oral administration (given as a single dose of 30 mg and dosed to steady-state with 30 mg t.i.d. for 6 days). The clinical response to these age-related pharmacokinetic differences, however, was not considered significant. (See PRECAUTIONS, Geriatric Use.)
Nonclinical Toxicology
In a two-year study, higher incidences of adenocarcinoma of the uterus and Leydig-cell adenoma of the testes were observed in rats given a diet containing 1800 ppm nimodipine (equivalent to 91 to 121 mg/kg/day nimodipine) than in placebo controls. The differences were not statistically significant, however, and the higher rates were well within historical control range for these tumors in the Wistar strain. Nimodipine was found not to be carcinogenic in a 91-week mouse study but the high dose of 1800 ppm nimodipine-in-feed (546 to 774 mg/kg/day) shortened the life expectancy of the animals. Mutagenicity studies, including the Ames, micronucleus and dominant lethal tests were negative. Nimodipine did not impair the fertility and general reproductive performance of male and female Wistar rats following oral doses of up to 30 mg/kg/day when administered daily for more than 10 weeks in the males and 3 weeks in the females prior to mating and continued to day 7 of pregnancy. This dose in a rat is about 4 times the equivalent clinical dose of 60 mg q4h in a 50 kg patient.
Clinical Studies
Clinical Studies (capsule)
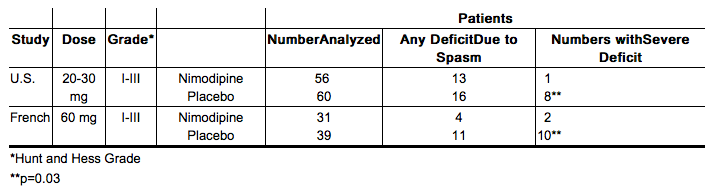
Nimodipine has been shown, in 4 randomized, double-blind, placebo-controlled trials, to reduce the severity of neurological deficits resulting from vasospasm in patients who have had a recent subarachnoid hemorrhage (SAH). The trials used doses ranging from 20-30 mg to 90 mg every 4 hours, with drug given for 21 days in 3 studies, and for at least 18 days in the other. Three of the four trials followed patients for 3-6 months. Three of the trials studied relatively well patients, with all or most patients in Hunt and Hess Grades I - III (essentially free of focal deficits after the initial bleed) the fourth studied much sicker patients, Hunt and Hess Grades III - V. Two studies, one U.S., one French, were similar in design, with relatively unimpaired SAH patients randomized to nimodipine or placebo. In each, a judgment was made as to whether any late-developing deficit was due to spasm or other causes, and the deficits were graded. Both studies showed significantly fewer severe deficits due to spasm in the nimodipine group; the second (French) study showed fewer spasm-related deficits of all severities. No effect was seen on deficits not related to spasm.
A third, large, study was performed in the United Kingdom in SAH patients with all grades of severity (but 89% were in Grades I-III). Nimodipine was dosed 60 mg every 4 hours. Outcomes were not defined as spasm related or not but there was a significant reduction in the overall rate of infarction and severely disabling neurological outcome at 3 months:
A Canadian study entered much sicker patients, (Hunt and Hess Grades III-V), who had a high rate of death and disability, and used a dose of 90 mg every 4 hours, but was otherwise similar to the first two studies. Analysis of delayed ischemic deficits, many of which result from spasm, showed a significant reduction in spasm-related deficits. Among analyzed patients (72 nimodipine, 82 placebo), there were the following outcomes.
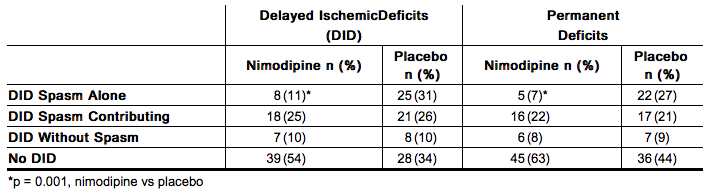
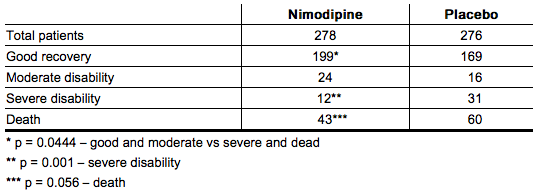
When data were combined for the Canadian and the United Kingdom studies, the treatment difference on success rate (i.e., good recovery) on the Glasgow Outcome Scale was 25.3% (nimodipine) versus 10.9% (placebo) for Hunt and Hess Grades IV or V. The table below demonstrates that nimodipine tends to improve good recovery of SAH patients with poor neurological status post-ictus, while decreasing the numbers with severe disability and vegetative survival.

A dose-ranging study comparing 30, 60 and 90 mg doses found a generally low rate of spasm-related neurological deficits but no dose response relationship.
Clinical Studies (solution)
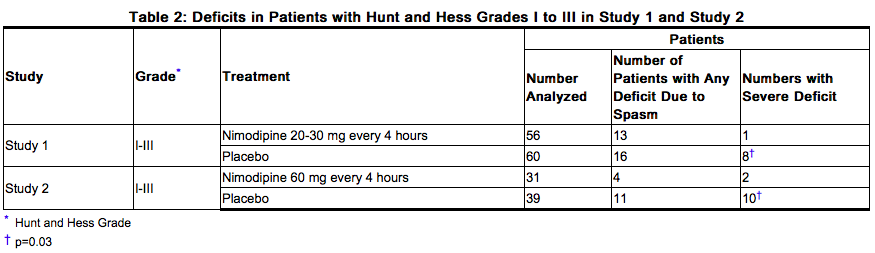
The safety and efficacy of Nimodipine (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH. Nimodipine (nimodipine oral solution) has comparable bioavailability to nimodipine oral capsules. Nimodipine has been shown in 4 randomized, double-blind, placebo-controlled trials to reduce the severity of neurological deficits resulting from vasospasm in patients who have had a recent SAH (Studies 1, 2, 3, and 4). The trials used doses ranging from 20-30 mg to 90 mg every 4 hours, with drug given for 21 days in 3 studies, and for at least 18 days in the other. Three of the four trials followed patients for 3-6 months. Three of the trials studied relatively well patients, with all or most patients in Hunt and Hess Grades I - III (essentially free of focal deficits after the initial bleed). Study 4 studied much sicker patients with Hunt and Hess Grades III - V. Studies 1 and 2 were similar in design, with relatively unimpaired SAH patients randomized to nimodipine or placebo. In each, a judgment was made as to whether any late-developing deficit was due to spasm or other causes, and the deficits were graded. Both studies showed significantly fewer severe deficits due to spasm in the nimodipine group; Study 2 showed fewer spasm-related deficits of all severities. No effect was seen on deficits not related to spasm. See Table 2.
Study 3 was a 554-patient trial that included SAH patients with all grades of severity (89% were in Hunt and Hess Grades I-III). In Study 3, patients were treated with placebo or 60 mg of nimodipine every 4 hours. Outcomes were not defined as spasm related or not but there was a significant reduction in the overall rate of brain infarction and severely disabling neurological outcome at 3 months (Table 3):
Study 4 enrolled much sicker patients, (Hunt and Hess Grades III-V), who had a high rate of death and disability, and used a dose of 90 mg every 4 hours, but was otherwise similar to Study 1 and Study 2. Analysis of delayed ischemic deficits, many of which result from spasm, showed a significant reduction in spasm-related deficits. Among analyzed patients (72 nimodipine, 82 placebo), there were the following outcomes (Table 4).

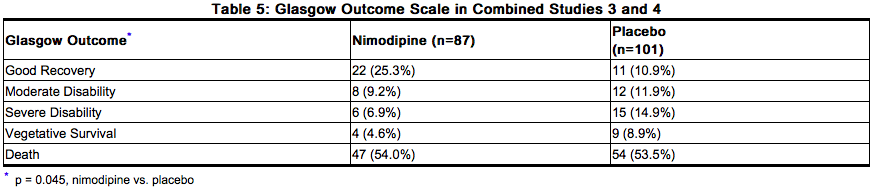
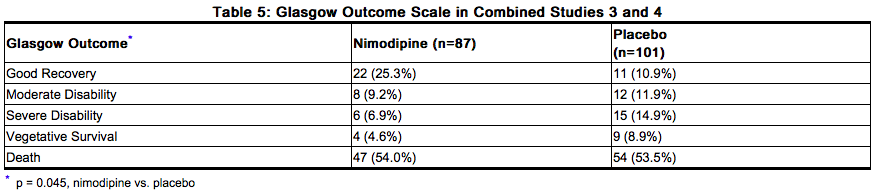
When data were combined for Study 3 and Study 4, the treatment difference on success rate (i.e., good recovery) on the Glasgow Outcome Scale was 25.3% (nimodipine) versus 10.9% (placebo) for Hunt and Hess Grades IV or V. Table 5 demonstrates that nimodipine tends to improve good recovery of SAH patients with poor neurological status post-ictus, while decreasing the numbers with severe disability and vegetative survival.
A dose-ranging study comparing 30 mg, 60 mg, and 90 mg doses found a generally low rate of spasm-related neurological deficits but no dose response relationship.
How Supplied
Nimodipine Capsules
30 mg - Oblong, white opaque, soft gelatin capsules in Unit Dose Package of 30 NDC 23155-108-30 Unit Dose Package of 100 NDC 23155-108-00
Printed H108 in black ink.
The capsules should be stored in the manufacturer’s original package.
Nimodipine Solution
- NDC 24338-200-16: 16 oz. bottle (473 mL)
- NDC 24338-200-12: Carton containing 12 individually wrapped packages. Each package contains one 20 mL Unit-Dose cup (NDC 24338-200-20) and one oral syringe.
Storage
Nimodipine Capsules
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature]. Capsules should be protected from light and freezing.
Nimodipine Solution
Store at 25ºC (77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Protect from light. Do not refrigerate.
Images
Drug Images
{{#ask: Page Name::Nimodipine sandbox |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nimodipine sandbox |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Inform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure [see Warnings and Precautions (5.1)]. Inform them that use of Nimodipine with anti-hypertensives can cause increased drop in blood pressure [see Drug Interactions (7.1)]. Patients should be aware that ingestion of grapefruit or grapefruit juice should be avoided when taking Nimodipine due to its ability to increase nimodipine plasma concentrations and potential to increase the risk of hypotension [see Drug Interactions (7.2)]. Pregnant women should be advised that a harmful effect of Nimodipine on the fetus cannot be ruled out and the drug should only be used if the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations, Pregnancy (8.1)]. Manufactured for: arbor™ PHARMACEUTICALS, LLC. Atlanta, GA 30328 Manufactured by: Importfab Pointe-Claire, QC, Canada H9R 1C9 Distributed By Arbor Pharmaceuticals, LLC., Atlanta, GA 30328 Nimodipine is a trademark of Arbor Pharmaceuticals, LLC © 2013 Arbor Pharmaceuticals, LLC NIM-PI-02 PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label NDC: 24338-200-16 16 oz. (473 mL) Nimodipine® (nimodipine) oral solution 60 mg/20 mL For Oral Use Only Distributed by: arbor™ PHARMACEUTICALS, LLC. Atlanta, GA 30328
Precautions with Alcohol
Alcohol-Nimodipine sandbox interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Nimotop
- Nimodipine
Look-Alike Drug Names
Nimodipine - Nicardipine Nimodipine - Nifedipine[5]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ de Carolis P, de Capoa D, Agati R, Baldrati A, Sacquegna T (1988). "Episodic cluster headache: short and long term results of prophylactic treatment". Headache. 28 (7): 475–6. PMID 3149629.
- ↑ Meyer JS, Hardenberg J (1983). "Clinical effectiveness of calcium entry blockers in prophylactic treatment of migraine and cluster headaches". Headache. 23 (6): 266–77. PMID 6358126.
- ↑ Fritze J, Walden J (1995). "Clinical findings with nimodipine in dementia: test of the calcium hypothesis". J Neural Transm Suppl. 46: 439–53. PMID 8821080.
- ↑ Ban TA, Morey L, Aguglia E, Azzarelli O, Balsano F, Marigliano V; et al. (1990). "Nimodipine in the treatment of old age dementias". Prog Neuropsychopharmacol Biol Psychiatry. 14 (4): 525–51. PMID 2236581.
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Nimodipine sandbox |Pill Name=No_image.jpg |Drug Name=nimodipine capsule, liquid filled |Pill Ingred=glycerin, ferrosoferric oxide, hypromelloses, gelatin, peppermint oil, polyethylene glycols, mannitol, propylene glycol, sorbitol, titanium dioxide|+sep=; |Pill Imprint=H108 |Pill Dosage=30 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=24.00 |Pill Scoring=1 |Pill Image= |Drug Author=Heritage |NDC=23155-108
}}
{{#subobject:
|Label Page=Nimodipine sandbox |Label Name=Nimodipine_label_01.jpg
}}
{{#subobject:
|Label Page=Nimodipine sandbox |Label Name=Nimodipine_label_02.jpg
}}
{{#subobject:
|Label Page=Nimodipine sandbox |Label Name=Nimodipine_panel_01.png
}}
{{#subobject:
|Label Page=Nimodipine sandbox |Label Name=Nimodipine_panel_02.png
}}
