Nexterone injection dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage and Administration
Amiodarone shows considerable interindividual variation in response. Although a starting dose adequate to suppress life-threatening arrhythmias is needed, close monitoring with adjustment of dose is essential. The recommended starting dose of nexterone is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen:
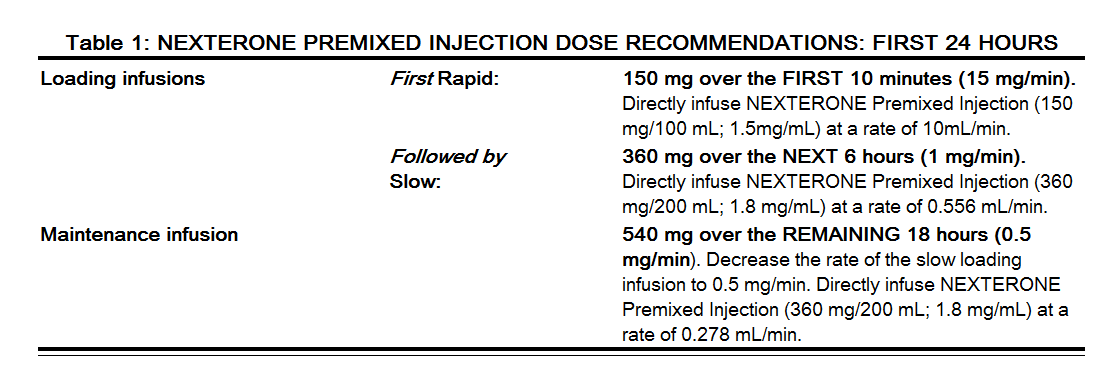 |
After the first 24 hours, continue the maintenance infusion rate of 0.5 mg/min (720 mg per 24 hours) by directly infusing nexterone Premixed Injection (360 mg/200 mL; 1.8 mg/mL) at a rate of 0.278 mL/min. The rate of the maintenance infusion may be increased to achieve effective arrhythmia suppression.
In the event of breakthrough episodes of VF or hemodynamically unstable VT, use 150 mg supplemental infusions of nexterone (infused over 10 minutes to minimize the potential for hypotension).
The first 24-hour dose may be individualized for each patient; however, in controlled clinical trials, mean daily doses above 2100 mg were associated with an increased risk of hypotension. Do not exceed an initial infusion rate of 30 mg/min.
Based on the experience from clinical studies of intravenous amiodarone, a maintenance infusion of up to 0.5 mg/min can be continued for 2 to 3 weeks regardless of the patient's age, renal function, or left ventricular function. There has been limited experience in patients receiving intravenous amiodarone for longer than 3 weeks.
Administer nexterone, whenever possible, through a central venous catheter dedicated to that purpose. Use an in-line filter during administration.
Intravenous amiodarone loading infusions at much higher concentrations and rates of infusion much faster than recommended have resulted in hepatocellular necrosis and acute renal failure, leading to death [see Warnings and Precautions (5.3)].
Intravenous amiodarone concentrations greater than 3 mg/mL have been associated with a high incidence of peripheral vein phlebitis; however, concentrations of 2.5 mg/mL or less appear to be less irritating. Therefore, for infusions longer than 1 hour, do not exceed nexterone concentrations of 2 mg/mL, unless a central venous catheter is used [see Adverse Reactions (6.2)].
Nexterone Premixed Injection is available in GALAXY containers as a single-use, ready-to-use, iso-osmotic solution in dextrose for intravenous administration. No further dilution is required. nexterone Premixed Injection should not be combined with any product in the same intravenous line or premixed container. Protect from light until ready to use.
Nexterone does not need to be protected from light during administration.
Since the premixed container is for single-use only, any unused portion should be discarded.
Note: Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. Check for minute leaks prior to use by squeezing the bag firmly. If leaks are detected, discard solution as sterility may be impaired.
Caution: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.
Preparation of nexterone Premixed Injection for administration:
Suspend container from eyelet support. Remove protector from outlet port at bottom of container. Attach administration set. Refer to complete directions accompanying set.
Admixture Incompatibility
Nexterone in D5W is incompatible with the drugs shown in Table 2.
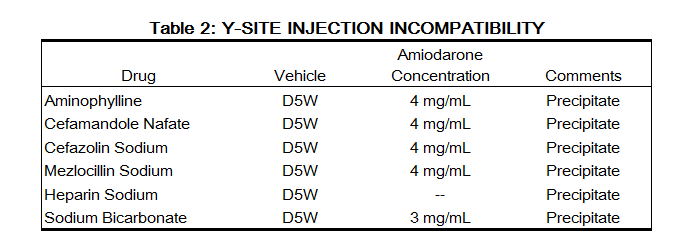 |
Intravenous to Oral Transition Patients whose arrhythmias have been suppressed by nexterone may be switched to oral amiodarone. The optimal dose for changing from intravenous to oral administration of amiodarone will depend on the dose of nexterone already administered, as well as the bioavailability of oral amiodarone. When changing to oral amiodarone therapy, clinical monitoring is recommended, particularly for elderly patients. See package insert for oral amiodarone.
Since grapefruit juice is known to inhibit CYP3A-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone, do not drink grapefruit juice during treatment with oral amiodarone [see Drug Interactions (7)].
Table 3 provides suggested doses of oral amiodarone to be initiated after varying durations of nexterone administration. These recommendations are made on the basis of a similar total body amount of amiodarone delivered by the intravenous and oral routes, based on 50% bioavailability of oral amiodarone.[1]
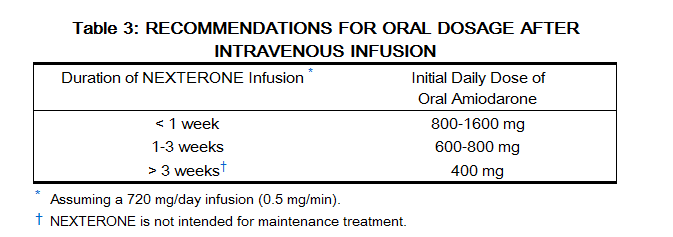 |
References
Adapted from the FDA Package Insert.