Micafungin dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage and Administration
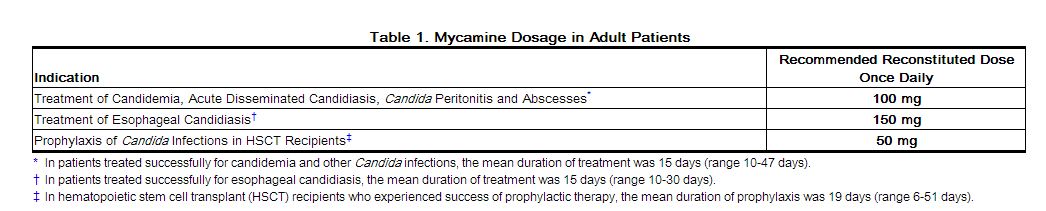
Do not mix or co-infuse Mycamine with other medications. Mycamine has been shown to precipitate when mixed directly with a number of other commonly used medications. The recommended doses for adult patients based on indications are shown in Table 1.
Dose and Schedule for Adults
 |
A loading dose is not required. Typically, 85% of the steady-state concentration is achieved after three daily Mycamine doses.
No dosing adjustments are required based on race, gender, or in patients with severe renal impairment or in patients with mild, moderate, or severe hepatic impairment.
No dose adjustment for Mycamine is required with concomitant use of mycophenolate mofetil, cyclosporine, tacrolimus, prednisolone, sirolimus, nifedipine, fluconazole, voriconazole, itraconazole, amphotericin B, ritonavir, or rifampin.
Dose and Schedule for Pediatric Patients
The recommended doses for pediatric patients based on indication and weight are shown in Table 2.
 |
Directions for Reconstitution, Dilution, and Preparation
Please read this entire section carefully before beginning reconstitution.
Reconstitution
Reconstitute Mycamine vials by aseptically adding 5 mL of one of the following compatible solutions:
• 0.9% Sodium Chloride Injection, USP (without a bacteriostatic agent).
• 5% Dextrose Injection, USP
To minimize excessive foaming, Gently dissolve the Mycamine powder by swirling the vial. Do Not Vigorously Shake The Vial. Visually inspect the vial for particulate matter.
Mycamine 50 mg vial: after reconstitution each mL contains 10 mg of micafungin sodium. Mycamine 100 mg vial: after reconstitution each mL contains 20 mg of micafungin sodium.
As with all parenteral drug products, reconstituted Mycamine should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use material if there is any evidence of precipitation or foreign matter. Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in Mycamine or in the materials specified for reconstitution and dilution.
Dilution and Preparation
The diluted solution should be protected from light. It is not necessary to cover the infusion drip chamber or the tubing.
Adult Patients
1. Add the appropriate volume of reconstituted Mycamine into 100 mL of 0.9% Sodium Chloride Injection, USP or 100 mL of 5% Dextrose Injection, USP.
2. Appropriately label the bag.
Pediatric Patients
1. Calculate the total Mycamine dose in milligrams (mg) by multiplying the recommended pediatric dose (mg/kg) for a given indication [seeTable 2] and the weight of the patient in kilograms (kg).
2. To calculate the volume (mL) of drug needed, divide the calculated dose (mg) from step 1 by the final concentration of the selected reconstituted vial(s) (either 10 mg/mL for the 50 mg vial or 20 mg/mL for the 100 mg vial), see example below:
Using 50 mg vials Divide the calculated mg dose (from step 1) by 10 mg/mL to determine the volume (mL) needed.
OR
Using 100 mg vials Divide the calculated mg dose (from step 1) by 20 mg/mL to determine the volume (mL) needed.
3. Withdraw the calculated volume (mL) of drug needed from the selected concentration and size of reconstituted Mycamine vial(s) used in Step 2 (ensure the selected concentration and vial size used to calculate the dose is also used to prepare the infusion).
4. Add the withdrawn volume of drug (step 3) to a 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP intravenous infusion bag or syringe. Ensure that the final concentration of the solution is between 0.5 mg/mL to 4 mg/mL.
Note: To minimize the risk of infusion reactions, concentrations of greater than 1.5 mg/mL should be administered via central catheter.
5. Appropriately label the infusion bag or syringe. For concentrations above 1.5 mg/mL, if required, label to specifically warn to administer the solution via central catheter.
Mycamine is preservative-free. Discard partially used vials.
Infusion Volume and Duration
Mycamine should be administered by intravenous infusion only. Infuse over one hour. More rapid infusions may result in more frequent histamine mediated reactions.
An existing intravenous line should be flushed with 0.9% Sodium Chloride Injection, USP, prior to infusion of Mycamine.
Pediatric Patients
Mycamine should be infused over one hour. To minimize the risk of infusion reactions, concentrations of greater than 1.5 mg/mL should be administered via central catheter.[1]
References
Adapted from the FDA Package Insert.