Methoxsalen (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shanshan Cen, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
Methoxsalen with UV radiation should be used only by physicians who have special competence in the diagnosis and treatment of psoriasis and vitiligo and who have special training and experience in photochemotherapy. Psoralen and ultraviolet radiation therapy should be under constant supervision of such a physician. For the treatment of patients with psoriasis, photochemotherapy should be restricted to patients with severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, and only when the diagnosis is certain. Because of the possibilities of ocular damage, aging of the skin, and skin cancer (including melanoma), the patient should be fully informed by the physician of the risks inherent in this therapy. When methoxsalen is used in combination with photopheresis, refer to the UVAR* System Operator's Manual for specific warnings, cautions, indications, and instructions related to photopheresis.
CAUTION: 8-MOP® Capsules (Methoxsalen Hard Gelatin Capsules) may not be interchanged with Oxsoralen-Ultra® Capsules (Methoxsalen Soft Gelatin Capsules) without retitration of the patient.
|
Overview
Methoxsalen (oral) is a naturally occurring photoactive substance that is FDA approved for the treatment of severe, recalcitrant, disabling psoriasis not adequately responsive to other forms of therapy and when the diagnosis has been supported by biopsy. It should be used with long wave ultraviolet radiation. There is a Black Box Warning for this drug as shown here. Common adverse reactions include erythema, pain of skin, pruritus, nausea, dizziness, headache, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
A. Photochemotherapy (methoxsalen with long wave UVA radiation) is indicated for the symptomatic control of severe, recalcitrant, disabling psoriasis not adequately responsive to other forms of therapy and when the diagnosis has been supported by biopsy. Photochemotherapy is intended to be administered only in conjunction with a schedule of controlled doses of long wave ultraviolet radiation.
B. Photochemotherapy (methoxsalen with long wave ultraviolet radiation) is indicated for the repigmentation of idiopathic vitiligo.
C. Photopheresis (methoxsalen with long wave ultraviolet radiation of white blood cells) is indicated for use with the UVAR* System in the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma (CTCL) in persons who have not been responsive to other forms of treatment. While this dosage form of methoxsalen has been approved for use in combination with photopheresis. Oxsoralen Ultra® Capsules have not been approved for that use.
Dosage
A. VITILIGO THERAPY 1. DRUG DOSAGE:
Two capsules (10 mg each) in one dose taken with milk or in food two to four hours before ultraviolet light exposure.
2. LIGHT EXPOSURE:
The exposure time to sunlight should comply with the following guide:

Subsequent Exposure: Gradually increase exposure based on erythema and tenderness of the amelanotic skin.
Therapy should be on alternate days and never two consecutive days.
B. psoriasis THERAPY 1. DRUG DOSAGE - INITIAL THERAPY:
The methoxsalen capsules should be taken 2 hours before UVA exposure with some food or milk according to the following table:

Additional drug dosage directions are as follows:
a. Weight Change: In the event that the weight of a patient changes during treatment such that he/she falls into an adjacent weight range/dose category, no change in the dose of methoxsalen is usually required. If, in the physician's opinion, however, a weight change is sufficiently great to modify the drug dose, then an adjustment in the time of exposure to UVA should be made.
b. Dose/Week: The number of doses per week of methoxsalen capsules will be determined by the patient's schedule of UVA exposures. In no case should treatments be given more often than once every other day because the full extent of phototoxic reactions may not be evident until 48 hours after each exposure.
c. Dosage Increase: Dosage may be increased by 10 mg. after the fifteenth treatment under the conditions outlined in section XI.B.4.b.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methoxsalen (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methoxsalen (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Methoxsalen (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methoxsalen (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methoxsalen (oral) in pediatric patients.
Contraindications
A. Patients exhibiting idiosyncratic reactions to psoralen compounds.
B. Patients possessing a specific history of light sensitive disease states should not initiate methoxsalen therapy. Diseases associated with photosensitivity include lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism.
C. Patients exhibiting melanoma or possessing a history of melanoma.
D. Patients exhibiting invasive squamous cell carcinomas.
E. Patients with aphakia, because of the significantly increased risk of retinal damage due to the absence of lenses.
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
Methoxsalen with UV radiation should be used only by physicians who have special competence in the diagnosis and treatment of psoriasis and vitiligo and who have special training and experience in photochemotherapy. Psoralen and ultraviolet radiation therapy should be under constant supervision of such a physician. For the treatment of patients with psoriasis, photochemotherapy should be restricted to patients with severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, and only when the diagnosis is certain. Because of the possibilities of ocular damage, aging of the skin, and skin cancer (including melanoma), the patient should be fully informed by the physician of the risks inherent in this therapy. When methoxsalen is used in combination with photopheresis, refer to the UVAR* System Operator's Manual for specific warnings, cautions, indications, and instructions related to photopheresis.
CAUTION: 8-MOP® Capsules (Methoxsalen Hard Gelatin Capsules) may not be interchanged with Oxsoralen-Ultra® Capsules (Methoxsalen Soft Gelatin Capsules) without retitration of the patient.
|
A. SKIN BURNING
Serious burns from either UVA or sunlight (even through window glass) can result if the recommended dosage of the drug and/or exposure schedules are not maintained.
B. CARCINOGENICITY
1. ANIMAL STUDIES
Topical or intraperitoneal methoxsalen has been reported to be a potent photocarcinogen in albino mice and hairless mice. However, methoxsalen given by the oral route to albino mice or by any route in pigmented mice is considerably less phototoxic or carcinogenic (Hakim et at. 196011; Pathak et al. 195912).
2. HUMAN STUDIES
A prospective study of 1380 patients over 5 years revealed an approximately nine-fold increase in risks of squamous cell carcinoma among PUVA treated patients (Stern et al. 197913 and Stern et al. 198014). This increase in risk appears greatest among patients who are fair skinned or had pre-PUVA exposure to 1) prolonged tar and UVB treatment, 2) ionizing radiation, or 3) arsenic.
In addition, an approximately two-fold increase in the risk of basal cell carcinoma was noted in this study. Roenigk et al. 198015 studied 690 patients for up to 4 years and found no increase in the risk of non-melanoma skin cancer. However, patients in this cohort had significantly less exposure to PUVA than in the Stern et al study. Recent analysis of new data in the Stern et al cohort (Stern et al., 199716) has shown that these patients had an elevated relative risk of contracting melanoma. The relative risk for melanoma in these patients was 2.3 (95 percent confidence interval 1.1 to 4.1). The risk is particularly higher in those patients who have received more than 250 PUVA treatments and in those whose treatment has spanned greater than 15 years earlier. Some patients developing melanoma did so even after having ceased PUVA therapy over 5 years earlier. These observations indicate the need for monitoring of PUVA patients for skin tumors throughout their lives.
In a study in Indian patients treated for 4 years for vitiligo, 12 percent developed keratoses, but not cancer, in the depigmented, vitiliginous areas (Mosher, 198017). Clinically, the keratoses were keratotic papules, actinic keratosis-like macules, nonscaling dome-shaped papules, and lichenoid porokeratotic-like papules.
C. CATARACTOGENICITY
1. ANIMAL STUDIES
Exposure to large doses of UVA causes cataracts in animals, and this effect is enhanced by the administration of methoxsalen (Cloud et al. 196018; Cloud et al. 196119; Freeman et al. 196920).
2. HUMAN STUDIES
It has been found that the concentration of methoxsalen in the lens is proportional to the serum level. If the lens is exposed to UVA during the time methoxsalen is present in the lens, photochemical action may lead to irreversible binding of methoxsalen to proteins and the DNA components of the lens (Lerman et al. 198021). However, if the lens is shielded from UVA, the methoxsalen will diffuse out of the lens in a 24 hour period21. Patients should be told emphatically to wear UVA-absorbing, wraparound sunglasses for the twenty-four (24) hour period following ingestion of methoxsalen, whether exposed to direct or indirect sunlight in the open or through a window glass.
Among patients using proper eye protection, there is no evidence for a significantly increased risk of cataracts in association with PUVA therapy.13 Thirty-five of 1380 patients have developed cataracts in the five years since their first PUVA treatment. This incidence is comparable to that expected in a population of this size and age distribution. No relationship between PUVA dose and cataract risk in this group has been noted.
D. ACTINIC DEGENERATION
Exposure to sunlight and/or ultraviolet radiation may result in "premature aging" of the skin.
E. BASAL CELL CARCINOMAS
Patients exhibiting multiple basal cell carcinomas or having a history of basal cell carcinomas should be diligently observed and treated.
F. RADIATION THERAPY
Patients having a history of previous x-ray therapy or grenz ray therapy should be diligently observed for signs of carcinoma.
G. ARSENIC THERAPY
Patients having a history of previous arsenic therapy should be diligently observed for signs of carcinoma.
H. HEPATIC DISEASES
Patients with hepatic insufficiency should be treated with caution since hepatic biotransformation is necessary for drug urinary excretion.
I. CARDIAC DISEASES
Patients with cardiac diseases or others who may be unable to tolerate prolonged standing or exposure to heat stress should not be treated in a vertical UVA chamber.
J. TOTAL DOSAGE
The total cumulative dose of UVA that can be given over long periods of time with safety has not as yet been established.
K. CONCOMITANT THERAPY
Special care should be exercised in treating patients who are receiving concomitant therapy (either topically or systemically) with known photosensitizing agents such as anthralin, coal tar or coal tar derivatives, griseofulvin, phenothiazines, nalidixic acid, fluoroquinolone antibiotics, halogenated salicylanilides (bacteriostatic soaps), sulfonamides, tetracyclines, thiazides, and certain organic staining dyes such as methylene blue, toluidine blue, rose bengal, and mythyl orange.
Adverse Reactions
Clinical Trials Experience
A. METHOXSALEN:
The most commonly reported side effect of methoxsalen alone is nausea, which occurs with approximately 10% of all patients. This effect may be minimized or avoided by instructing the patient to take methoxsalen with milk or food, or to divide the dose into two portions, taken approximately one-half hour apart. Other effects include nervousness, insomnia, and psychological depression.
B. COMBINED METHOXSALEN/UVA THERAPY:
1. pruritus:
This adverse reaction occurs with approximately 10% of all patients. In most cases, pruritus can be alleviated with frequent application of bland emollients or other topical agents; severe pruritus may require systemic treatment. If pruritus is unresponsive to these measures, shield pruritic areas from further UVA exposure until the condition resolves. If intractable pruritus is generalized, UVA treatment should be discontinued until the pruritus disappears.
2. erythema:
Mild, transient erythema at 24-48 hours after PUVA therapy is an expected reaction and indicates that a therapeutic interaction between methoxsalen and UVA occurred. An area showing moderate erythema (greater than Grade 2 – See TABLE 1 for grades of erythema) should be shielded during subsequent UVA exposures until the erythema has resolved. erythema greater than Grade 2 which appears within 24 hours after UVA treatment may signal a potentially severe burn. erythema may become progressively worse over the next 24 hours, since the peak erythemal reaction characteristically occurs 48 hours or later after methoxsalen ingestion. The patient should be protected from further UVA exposures and sunlight, and should be monitored closely.
3. IMPORTANT DIFFERENCES BETWEEN PUVA erythema AND SUNBURN:
PUVA-induced inflammation differs from sunburn or UVB phototherapy in several ways. The in situ depth of photochemistry is deeper within the tissue because UVA is transmitted further into the skin. The DNA lesions induced by PUVA are very different from UV-induced thymine dimers and may lead to a DNA crosslink. This DNA lesion may be more problematic to the cell because crosslinks are more lethal and psoralen-DNA photoproducts may be "new" or unfamiliar substrates for DNA repair enzymes. DNA synthesis is also suppressed longer after PUVA. The time course of delayed erythema is different with PUVA and may not involve the usual mediators seen in sunburn. PUVA-induced redness may be just beginning at 24 hours, when UVB erythema has already passed its peak. The erythema dose-response curve is also steeper for PUVA. Compared to equally erythemogenic doses of UVB, the histologic alterations induced by PUVA show more dermal vessel damage and longer duration of epidermal and dermal abnormalities.
4. OTHER ADVERSE REACTIONS:
Those reported include edema, dizziness, headache, malaise, depression, hypopigmentation, vesiculation and bullae formation, non-specific rash, herpes simplex, miliaria, urticaria, folliculitis, gastrointestinal disturbances, cutaneous tenderness, leg cramps, hypotension, and extension of psoriasis.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Methoxsalen (oral) in the drug label.
Drug Interactions
See WARNINGS Section.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with methoxsalen. It is also not known whether methoxsalen can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methoxsalen should be given to a woman only if clearly needed.
Pregnancy Category (AUS): B2
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methoxsalen (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methoxsalen (oral) during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methoxsalen is administered to a nursing woman.
Pediatric Use
Safety in children has not been established.
Geriatic Use
There is no FDA guidance on the use of Methoxsalen (oral) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Methoxsalen (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methoxsalen (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methoxsalen (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methoxsalen (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methoxsalen (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methoxsalen (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Methoxsalen (oral) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Methoxsalen (oral) in the drug label.
Overdosage
In the event of methoxsalen overdosage, induce emesis and keep the patient in a darkened room for at least 24 hours. Emesis is beneficial only within the first 2 to 3 hours after ingestion of methoxsalen, since maximum blood levels are reached by this time.
Pharmacology
| |
Methoxsalen (oral)
| |
| Systematic (IUPAC) name | |
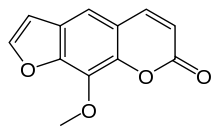
| 9-methoxy-7H-furo[3,2-g]chromen-7-one | |
| Identifiers | |
| CAS number | |
| ATC code | D05 D05BA02 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 216.19 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ~2 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
The combination treatment regimen of psoralen (P) and ultraviolet radiation of 320-400 nm wavelength commonly referred to as UVA is known by the acronym, PUVA. Skin reactivity to UVA (320-400 nm) radiation is markedly enhanced by the ingestion of methoxsalen. The drug reaches its maximum bioavailability 1 1/2-3 hours after oral administration and may last for up to 8 hours (Pathak et al., 1974)1. Methoxsalen is reversibly bound to serum albumin and is also preferentially taken up by epidermal cells (Artuc et al. 1979)2. At a dose which is six times larger than that used in humans, it induces mixed function oxidases in the liver of mice (Mandula et al. 1978)3. In both mice and man, methoxsalen is rapidly metabolized. Approximately 95% of the drug is excreted as a series of metabolites in the urine within 24 hours (Pathak et al. 1977)4.
The exact mechanism of action of methoxsalen with the epidermal melanocyctes and keratinocytes is not known. The best known biochemical reaction of methoxsalen is with DNA. Methoxsalen, upon photoactivation, conjugates and forms covalent bonds with DNA which leads to the formation of both monofunctional (addition to a single strand of DNA) and bifunctional adducts (crosslinking of psoralen to both strands of DNA) (Dall' Acqua et at., 19715; Cole, 19706; Musajo et al., 19747; Dall' Acqua et al., 19798). Reactions with proteins have also been described (Yoshikawa, et al., 19799).
Structure
8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds known as psoralens, or furocoumarins. The chemical name of methoxsalen is 9-methoxy-7 H-furo[3,2-g][1]-benzopyran-7-one; it has the following structure:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Methoxsalen (oral) in the drug label.
Pharmacokinetics
Methoxsalen acts as a photosensitizer. Administration of the drug and subsequent exposure to UVA can lead to cell injury. Orally administered methoxsalen reaches the skin via the blood and UVA penetrates well into the skin. If sufficient cell injury occurs in the skin, an inflammatory reaction occurs. The most obvious manifestation of this reaction is delayed erythema, which may not begin for several hours and peaks at 48-72 hours. The inflammation is followed, over several days to weeks, by repair which is manifested by increased melanization of the epidermis and thickening of the stratum corneum. The mechanisms of therapy are not known. In the treatment of vitiligo, it has been suggested that melanocytes in the hair follicle are stimulated to move up the follicle and to repopulate the epidermis (Ortonne et al. 197910). In the treatment of psoriasis, the mechanism is most often assumed to be DNA photodamage and resulting decrease in cell proliferation but other vascular, leukocyte, or cell regulatory mechanisms may also be playing some role. psoriasis is a hyperproliferative disorder and other agents known to be therapeutic for psoriasis are known to inhibit DNA synthesis.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Methoxsalen (oral) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Methoxsalen (oral) in the drug label.
How Supplied
8-MOP Capsules, each containing 10 mg of methoxsalen (8-methoxypsoralen) are available in pink-colored hard gelatin capsules in amber glass bottles of 50 (NDC 0187-0651-42), with ICN imprinted on the cap of the capsule and 600 imprinted on the body of the capsule.
Storage
Store at 25°C (77°F); excursions permitted to 15°C- 30°C (59°F- 86°F).
Images
Drug Images
{{#ask: Page Name::Methoxsalen (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Methoxsalen (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
This brochure is intended to provide you with information about the treatment of vitiligo and psoriasis. The entire brochure should be read so that you are aware of the requirements on your part to ensure the effectiveness and safety of the therapy. Any additional questions that you may have can be answered by your doctor or pharmacist. In addition, the pharmacist will have a copy of a very technical brochure entitled the "Physician's Package Insert" that you may wish to read.
What Is 8-MOP® (Methoxsalen)? 8-MOP® (methoxsalen) is a drug which has been shown to be effective in the treatment of certain skin diseases when combined with exposure to a very specific kind of light. The skin diseases are vitiligo and psoriasis. In either skin disease, the use of the drug must be combined with exposure to the special light to produce effective therapy.
What Is The Special Light? Light is classified into many different parts. One part is known as ultraviolet light, which is a normal component of sunlight. Artificial or man-made light sources are now available that produce the special part of light (ultraviolet "A") necessary for the most effective therapy.
What Is "PUVA"? "PUVA" is the name of the treatment for psoriasis and stands for the use of Psoralen drug (8-MOP®) in combination with UltraViolet A light.
What Is Vitiligo? Skin color is determined by the amount of a pigment called melanin in the skin. This pigment is formed by a normal chemical reaction in the skin which is promoted by ultraviolet light (for example, tanning). In vitiligo, some areas of the skin lose their ability to produce this pigment and patches appear that have less color than your normal skin. The combination of 8-MOP® and ultraviolet light helps restore the color to these areas.
Whats Is psoriasis? psoriasis is a skin condition with red and scaly patches. The cause of psoriasis is not known. PUVA (8-MOP® with ultraviolet A light) is used for the treatment of severe psoriasis that has not been helped by other methods of therapy.
What Should The Patient Do Before PUVA Therapy? Certain other medicines can make you more sensitive to the combination drug and light treatment. In addition, certain other medical conditions can be aggravated by this treatment. Before starting treatment, be sure to tell your doctor if you have experienced any of the folllowing:
had a severe reaction to 8-MOP ® in the past. had a recent x-ray treatment or are planning any. have or ever have had skin cancer. have or ever have had any eye problems such as cataracts or loss of the lens of the eyes. have or ever have had liver problems. have or ever have had heart or blood pressure problems. have any medical condition that requires you to stay out of the sun such as lupus erythematosus. are taking any drugs (either prescription or nonprescription). Some drugs can increase your sensitivity to ultraviolet light either from the sun or man-made sources. Examples of such drugs include major tranquilizers, sulfa drugs for the treatment of infection or diabetes, tetracycline antibiotics, griseofulvin products, thiazide-containing diuretics (blood pressure or water elimination drugs), and certain antibacterial or deodorant soaps. How Should The Patient Take 8-MOP®? The number of capsules recommended by your doctor should be taken with some food or milk according to the following schedule: For vitiligo - two to four hours before ultraviolet light treatment. For psoriasis - two hours before ultraviolet light treatment. 8-MOP ® is a potent drug. Never take more than is prescribed for you since it may result in burning and/or blistering of your skin after exposure to ultraviolet light. What Precautions Should Be Taken During And After PUVA Therapy? Eye Protection - Make sure that you wear special wrap-around sunglasses that totally block or absorb ultraviolet light. Put them on immediately after taking 8-MOP ® and continue wearing them for 24 hours if any light is present (even if indirect such as reflection or through window glass). Ordinary sunglasses are not adequate. Skin & Lip Protection - Do not allow exposure of your skin and lips to sunlight for 8 hours after treatment. In addition, do not expose your skin to either sunlight or sun lamps (regardless of safety claims) within 24 hours of a scheduled treatment. It is advisable to wear protective clothing (hat, gloves) to cover as much of your body as possible after treatment as well as using a sunscreen product having a protection factor of at least 15 (only use after treatment). How Long Will The Treatments Last? Vitiligo - May take from several months to several years to complete treatment. psoriasis - May take from six to eight weeks before lesions disappear. Maintenance treatments are usually needed to keep the disease under control. What Are The Problems Associated With Pregnancy Or Breast-Feeding? Birth control methods should be employed since the effects of PUVA therapy on the unborn child are not known. If you become pregnant, inform your doctor so that he can determine wheter it is necessary for you to temporarily stop therapy. Since it is not known whether 8-MOP ® passes into mother's milk, it is safer not to breast feed while taking this drug. What Are The Risks Of PUVA Therapy? Premature skin aging may result from prolonged PUVA therapy, especially with those individuals who tan poorly. This problem is similar to excessive exposure to sunlight. There is an increased risk of developing both melanoma and non-melanoma skin cancer. This risk is greater for individuals who fall into the following categories: fair skin that burns rather than tans. have had prior treatment with x-rays, grenz rays, or arsenic. have had coal tar and Ultra Violet B (UVB) treatment. Even though your doctor will be examining you, you should routinely and completely examine yourself for small growths on your skin or skin sores that will not heal. Immediately report such observations to your doctor.
Since studies have shown that animals with unprotected eyes have developed cataracts after PUVA therapy, you should have your eyes examined by an opthalmologist before starting PUVA therapy, after the first year of therapy, and every two years thereafter. What Are The Possible Side Effects? The most common side effects of PUVA therapy are nausea, itching, and redness of the skin. The use of milk or food when ingesting the drug may prevent the nausea. Tenderness or blistering of the skin may occur, but these symptoms can be helped by the use of skin products recommended by your doctor or pharmacist. Less frequent side effects include depression, dizziness, headache, swelling, rash, or leg cramps. Important: Contact your doctor if any side effect continues to bother you after 24-48 hours. What Else Should The Patient Know? Remember to take 8-MOP ® as directed by your doctor. If you forget to take the drug before your scheduled treatment, be sure to call your doctor to determine what he wishes you to do. Remember that the drug has been prescribed specifically for you and your diagnosed condition. Do not use the drug for any other conditions nor give the drug to others even if they have similar symptoms. If you think that you or anyone else has accidentally taken an overdose, stay out of the sunlight and immediately contact your poison control center, doctor, pharmacist, or nearest hospital emergency room. ALWAYS KEEP THIS DRUG AND ALL OTHER DRUGS OUT OF THE REACH OF CHILDREN. Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F).
Precautions with Alcohol
- Alcohol-Methoxsalen (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- OXSORALEN-ULTRA®[1]
Look-Alike Drug Names
- A® — B®[2]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "OXSORALEN-ULTRA- methoxsalen capsule, liquid filled".
- ↑ "http://www.ismp.org". External link in
|title=(help)