Medetomidine hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Medetomidine hydrochloride is a sedative that is FDA approved for the procedure of sedation of non-intubated patients prior to and/or during surgical and other procedures. Common adverse reactions include hypotension, bradycardia, and dry mouth.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Dosing Guidelines
- Dexmedetomidine hydrochloride injection dosing should be individualized and titrated to desired clinical response.
- Dexmedetomidine hydrochloride injection is not indicated for infusions lasting longer than 24 hours.
- Dexmedetomidine hydrochloride injection should be administered using a controlled infusion device.

Dosage Adjustment
Due to possible pharmacodynamic interactions, a reduction in dosage of dexmedetomidine hydrochloride injection or other concomitant anesthetics, sedatives, hypnotics or opioids may be required when co-administered. Dosage reductions may need to be considered for adult patients with hepatic impairment, and geriatric patients.
Preparation of Solution
- Strict aseptic technique must always be maintained during handling of dexmedetomidine hydrochloride injection.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Dexmedetomidine Hydrochloride Injection, Concentrate, 200 mcg/2 mL (100 mcg/mL)
- Dexmedetomidine hydrochloride injection must be diluted with 0.9% sodium chloride solution to achieve required concentration (4 mcg/mL) prior to administration. Preparation of solutions is the same, whether for the loading dose or maintenance infusion.
- To prepare the infusion, withdraw 2 mL of dexmedetomidine hydrochloride injection concentrate and add to 48 mL of 0.9% sodium chloride injection to a total of 50 mL. Shake gently to mix
Administration with Other Fluids
- Dexmedetomidine hydrochloride injection infusion should not be coadministered through the same intravenous catheter with blood or plasma because physical compatibility has not been established.
- Dexmedetomidine hydrochloride injection has been shown to be incompatible when administered with the following drugs: amphotericin B, diazepam.
Dexmedetomidine hydrochloride injection has been shown to be compatible when administered with the following intravenous fluids:
- 0.9% sodium chloride in water
- 5% dextrose in water
- 20% mannitol
- Lactated Ringer's solution
- 100 mg/mL magnesium sulfate solution
- 0.3% potassium chloride solution
Compatibility with Natural Rubber
Compatibility studies have demonstrated the potential for absorption of dexmedetomidine hydrochloride to some types of natural rubber. Although dexmedetomidine hydrochloride injection is dosed to effect, it is advisable to use administration components made with synthetic or coated natural rubber gaskets.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Medetomidine hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Medetomidine hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Medetomidine hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Medetomidine hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Medetomidine hydrochloride in pediatric patients.
Contraindications
None
Warnings
Drug Administration
Dexmedetomidine hydrochloride injection should be administered only by persons skilled in the management of patients in the operating room setting. Due to the known pharmacological effects of dexmedetomidine hydrochloride injection, patients should be continuously monitored while receiving dexmedetomidine hydrochloride injection.
Hypotension, Bradycardia, and Sinus Arrest
- Clinically significant episodes of bradycardia and sinus arrest have been reported with dexmedetomidine hydrochloride injection administration in young, healthy adult volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration.
- Reports of hypotension and bradycardia have been associated with dexmedetomidine hydrochloride injection infusion. If medical intervention is required, treatment may include decreasing or stopping the infusion of dexmedetomidine hydrochloride injection, increasing the rate of intravenous fluid administration, elevation of the lower extremities, and use of pressor agents. Because dexmedetomidine hydrochloride injection has the potential to augment bradycardia induced by vagal stimuli, clinicians should be prepared to intervene. The intravenous administration of anticholinergic agents (e.g., glycopyrrolate, atropine) should be considered to modify vagal tone. In clinical trials, glycopyrrolate or atropine were effective in the treatment of most episodes of dexmedetomidine hydrochloride-induced bradycardia. However, in some patients with significant cardiovascular dysfunction, more advanced resuscitative measures were required.
- Caution should be exercised when administering dexmedetomidine hydrochloride injection to patients with advanced heart block and/or severe ventricular dysfunction. Because dexmedetomidine hydrochloride injection decreases sympathetic nervous system activity, hypotension and/or bradycardia may be expected to be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension and in elderly patients.
- In clinical trials where other vasodilators or negative chronotropic agents were coadministered with dexmedetomidine hydrochloride injection an additive pharmacodynamic effect was not observed. Nonetheless, caution should be used when such agents are administered concomitantly with dexmedetomidine hydrochloride injection.
Transient Hypertension
- Transient hypertension has been observed primarily during the loading dose in association with the initial peripheral vasoconstrictive effects of dexmedetomidine hydrochloride injection. Treatment of the transient hypertension has generally not been necessary, although reduction of the loading infusion rate may be desirable.
Arousability
- Some patients receiving dexmedetomidine hydrochloride injection have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
Withdrawal
- In adult subjects, withdrawal symptoms were not seen after discontinuation of short term infusions of dexmedetomidine hydrochloride injection (< 6 hours).
Tolerance and Tachyphylaxis
- Use of dexmedetomidine beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose related increase in adverse reactions.
Hepatic Impairment
- Since dexmedetomidine clearance decreases with severity of hepatic impairment, dose reduction should be considered in patients with impaired hepatic function
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Use of dexmedetomidine hydrochloride injection has been associated with the following serious adverse reactions:
- Hypotension, bradycardia and sinus arrest.
- Transient hypertension
- Most common treatment-emergent adverse reactions, occurring in greater than 2% of patients in procedural sedation studies include hypotension, bradycardia and dry mouth.
Procedural Sedation
Adverse reaction information is derived from the two trials for procedural sedation in which 318 adult patients received dexmedetomidine hydrochloride injection. The mean total dose was 1.6 mcg/kg (range: 0.5 to 6.7), mean dose per hour was 1.3 mcg/kg/hr (range: 0.3 to 6.1) and the mean duration of infusion of 1.5 hours (range: 0.1 to 6.2). The population was between 18 to 93 years of age, 30% ≥ 65 years of age, 52% male and 61% Caucasian.
Treatment-emergent adverse reactions occurring at an incidence of > 2% are provided in Table 2. The most frequent adverse reactions were hypotension, bradycardia, and dry mouth. Pre-specified criteria for the vital signs to be reported as adverse reactions are footnoted below the table. The decrease in respiratory rate and hypoxia was similar between dexmedetomidine hydrochloride injection and comparator groups in both studies.

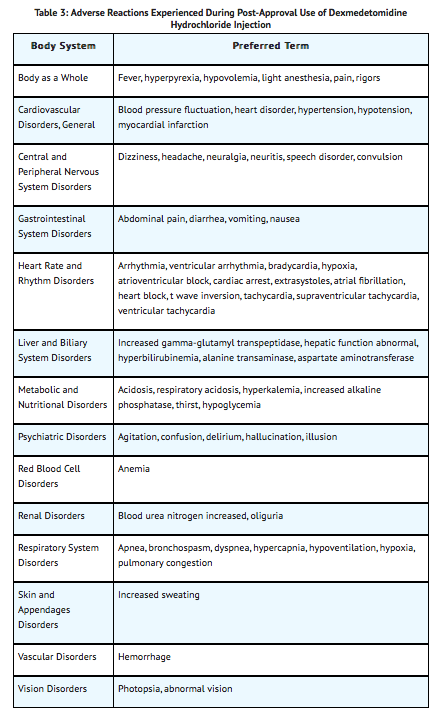
Postmarketing Experience
The following adverse reactions have been identified during post approval use of dexmedetomidine hydrochloride injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypotension and bradycardia were the most common adverse reactions associated with the use of dexmedetomidine hydrochloride injection during post approval use of the drug.
Drug Interactions
Anesthetics, Sedatives, Hypnotics, Opioids
Coadministration of dexmedetomidine hydrochloride injection with anesthetics, sedatives, hypnotics, and opioids is likely to lead to an enhancement of effects. Specific studies have confirmed these effects with sevoflurane, isoflurane, propofol, alfentanil, and midazolam. No pharmacokinetic interactions between dexmedetomidine hydrochloride injection and isoflurane, propofol, alfentanil and midazolam have been demonstrated. However, due to possible pharmacodynamic interactions, when coadministered with dexmedetomidine hydrochloride injection, a reduction in dosage of dexmedetomidine hydrochloride injection or the concomitant anesthetic, sedative, hypnotic or opioid may be required.
Neuromuscular Blockers
In one study of ten healthy adult volunteers, administration of dexmedetomidine hydrochloride injection for 45 minutes at a plasma concentration of one ng/mL resulted in no clinically meaningful increases in the magnitude of neuromuscular blockade associated with rocuronium administration
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well controlled studies of dexmedetomidine hydrochloride injection use in pregnant women. In an in vitro human placenta study, placental transfer of dexmedetomidine occurred. In a study in the pregnant rat, placental transfer of dexmedetomidine was observed when radiolabeled dexmedetomidine was administered subcutaneously. Thus, fetal exposure should be expected in humans, and dexmedetomidine hydrochloride injection should be used during pregnancy only if the potential benefits justify the potential risk to the fetus.
Teratogenic effects were not observed in rats following subcutaneous administration of dexmedetomidine during the period of fetal organogenesis (from gestation day 5 to 16) with doses up to 200 mcg/kg (representing a dose approximately equal to the maximum recommended human intravenous dose based on body surface area) or in rabbits following intravenous administration of dexmedetomidine during the period of fetal organogenesis (from gestation day 6 to 18) with doses up to 96 mcg/kg (representing approximately half the human exposure at the maximum recommended dose based on plasma area under the time-curve comparison). However, fetal toxicity, as evidenced by increased post-implantation losses and reduced live pups, was observed in rats at a subcutaneous dose of 200 mcg/kg. The no-effect dose in rats was 20 mcg/kg (representing a dose less than the maximum recommended human intravenous dose based on a body surface area comparison). In another reproductive toxicity study when dexmedetomidine was administered subcutaneously to pregnant rats at 8 and 32 mcg/kg (representing a dose less than the maximum recommended human intravenous dose based on a body surface area comparison) from gestation day 16 through weaning, lower offspring weights were observed. Additionally, when offspring of the 32 mcg/kg group were allowed to mate, elevated fetal and embryocidal toxicity and delayed motor development was observed in second generation offspring.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Medetomidine hydrochloride in women who are pregnant.
Labor and Delivery
The safety of dexmedetomidine hydrochloride injection during labor and delivery has not been studied.
Nursing Mothers
It is not known whether dexmedetomidine hydrochloride is excreted in human milk. Radiolabeled dexmedetomidine administered subcutaneously to lactating female rats was excreted in milk. Because many drugs are excreted in human milk, caution should be exercised when dexmedetomidine hydrochloride injection is administered to a nursing woman.
Pediatric Use
Safety and efficacy have not been established for Procedural Sedation in pediatric patients. Additional information describing clinical studies in a different indication in which efficacy was not demonstrated in pediatric patients is approved for Hospira’s dexmedetomidine injection. However, due to Hospira’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. The use of dexmedetomidine for procedural sedation in pediatric patients has not been evaluated.
Geriatic Use
A total of 131 patients in the clinical studies were 65 years of age and over. A total of 47 patients were 75 years of age and over. Hypotension occurred in a higher incidence in dexmedetomidine hydrochloride injection-treated patients 65 years or older (72%) and 75 years or older (74%) as compared to patients < 65 years (47%). A reduced loading dose of 0.5 mcg/kg given over 10 minutes is recommended and a reduction in the maintenance infusion should be considered for patients greater than 65 years of age.
Gender
There is no FDA guidance on the use of Medetomidine hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Medetomidine hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Medetomidine hydrochloride in patients with renal impairment.
Hepatic Impairment
Since dexmedetomidine clearance decreases with increasing severity of hepatic impairment, dose reduction should be considered in patients with impaired hepatic function.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Medetomidine hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Medetomidine hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Medetomidine hydrochloride Administration in the drug label.
Monitoring
There is limited information regarding Medetomidine hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Medetomidine hydrochloride and IV administrations.
Overdosage
The tolerability of dexmedetomidine hydrochloride injection was studied in one study in which healthy adult subjects were administered doses at and above the recommended dose of 0.2 to 0.7 mcg/kg/hr. The maximum blood concentration achieved in this study was approximately 13 times the upper boundary of the therapeutic range. The most notable effects observed in two subjects who achieved the highest doses were first degree atrioventricular block and second degree heart block. No hemodynamic compromise was noted with the atrioventricular block and the heart block resolved spontaneously within one minute.
One patient who received a loading bolus dose of undiluted dexmedetomidine hydrochloride injection (19.4 mcg/kg), had cardiac arrest from which he was successfully resuscitated.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Precedex, Dexdor |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intravenous infusion only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 94% |
| Metabolism | Near complete hepatic metabolism to inactive metabolites |
| Elimination half-life | 2 hours |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H16N2 |
| Molar mass | 200.28 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
Dexmedetomidine hydrochloride injection is a relatively selective alpha2-adrenergic agonist with sedative properties. Alpha2 selectivity is observed in animals following slow intravenous infusion of low and medium doses (10 to 300 mcg/kg). Both alpha1 and alpha2 activity is observed following slow intravenous infusion of high doses (≥ 1000 mcg/kg) or with rapid intravenous administration.
Structure
Dexmedetomidine hydrochloride has a molecular weight of 236.7 and the empirical formula is C13H16N2 ·• HCl and the structural formula is:

Pharmacodynamics
In a study in healthy volunteers (N = 10), respiratory rate and oxygen saturation remained within normal limits and there was no evidence of respiratory depression when dexmedetomidine hydrochloride injection was administered by intravenous infusion at doses within the recommended dose range (0.2 to 0.7 mcg/kg/hr).
Pharmacokinetics
Following intravenous administration, dexmedetomidine exhibits the following pharmacokinetic parameters: a rapid distribution phase with a distribution half-life (t1/2) of approximately 6 minutes; a terminal elimination half-life (t1/2) of approximately 2 hours; and steady-state volume of distribution (Vss) of approximately 118 liters. Clearance is estimated to be approximately 39 L/h. The mean body weight associated with this clearance estimate was 72 kg.
Dexmedetomidine exhibits linear pharmacokinetics in the dosage range of 0.2 to 0.7 mcg/kg/hr when administered by intravenous infusion for up to 24 hours. Table 4 shows the main pharmacokinetic parameters when dexmedetomidine hydrochloride injection was infused (after appropriate loading doses) at maintenance infusion rates of 0.17 mcg/kg/hr (target plasma concentration of 0.3 ng/mL) for 12 and 24 hours, 0.33 mcg/kg/hr (target plasma concentration of 0.6 ng/mL) for 24 hours, and 0.70 mcg/kg/hr (target plasma concentration of 1.25 ng/mL) for 24 hours.

Dexmedetomidine pharmacokinetic parameters after dexmedetomidine hydrochloride injection maintenance doses of 0.2 to 1.4 mcg/kg/hr for > 24 hours were similar to the PK parameters after dexmedetomidine hydrochloride injection maintenance dosing for < 24 hours in other studies. The values for clearance (CL), volume of distribution (V), and t1/2 were 39.4 L/hr, 152 L, and 2.67 hours, respectively.
Distribution
The steady-state volume of distribution (Vss) of dexmedetomidine was approximately 118 liters. Dexmedetomidine protein binding was assessed in the plasma of normal healthy male and female subjects. The average protein binding was 94% and was constant across the different plasma concentrations tested. Protein binding was similar in males and females. The fraction of dexmedetomidine that was bound to plasma proteins was significantly decreased in subjects with hepatic impairment compared to healthy subjects.
The potential for protein binding displacement of dexmedetomidine by fentanyl, ketorolac, theophylline, digoxin and lidocaine was explored in vitro, and negligible changes in the plasma protein binding of dexmedetomidine were observed. The potential for protein binding displacement of phenytoin, warfarin, ibuprofen, propranolol, theophylline and digoxin by dexmedetomidine was explored in vitro and none of these compounds appeared to be significantly displaced by dexmedetomidine.
Metabolism
Dexmedetomidine undergoes almost complete biotransformation with very little unchanged dexmedetomidine excreted in urine and feces. Biotransformation involves both direct glucuronidation as well as cytochrome P450 mediated metabolism. The major metabolic pathways of dexmedetomidine are: direct N-glucuronidation to inactive metabolites; aliphatic hydroxylation (mediated primarily by CYP2A6) of dexmedetomidine to generate 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxy-dexmedetomidine, and 3-carboxy-dexmedetomidine; and N-methylation of dexmedetomidine to generate 3-hydroxy N-methyl-dexmedetomidine, 3-carboxy N-methyl-dexmedetomidine, and dexmedetomidine-N-methyl O-glucuronide.
Elimination
The terminal elimination half-life (t1/2) of dexmedetomidine is approximately 2 hours and clearance is estimated to be approximately 39 L/h. A mass balance study demonstrated that after nine days an average of 95% of the radioactivity, following intravenous administration of radiolabeled dexmedetomidine, was recovered in the urine and 4% in the feces. No unchanged dexmedetomidine was detected in the urine. Approximately 85% of the radioactivity recovered in the urine was excreted within 24 hours after the infusion. Fractionation of the radioactivity excreted in urine demonstrated that products of N-glucuronidation accounted for approximately 34% of the cumulative urinary excretion. In addition, aliphatic hydroxylation of parent drug to form 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxy-dexmedetomidine, and 3-carboxylic acid-dexmedetomidine together represented approximately 14% of the dose in urine. N-methylation of dexmedetomidine to form 3-hydroxy N-methyl dexmedetomidine, 3-carboxy N-methyl dexmedetomidine, and N methyl O glucuronide dexmedetomidine accounted for approximately 18% of the dose in urine. The N Methyl metabolite itself was a minor circulating component and was undetected in urine. Approximately 28% of the urinary metabolites have not been identified.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenicity studies have not been performed with dexmedetomidine.
Dexmedetomidine was not mutagenic in vitro, in either the bacterial reverse mutation assay (E. coli and Salmonella typhimurium) or the mammalian cell forward mutation assay (mouse lymphoma). Dexmedetomidine was clastogenic in the in vitro human lymphocyte chromosome aberration test with, but not without, rat S9 metabolic activation. In contrast, dexmedetomidine was not clastogenic in the in vitro human lymphocyte chromosome aberration test with or without human S9 metabolic activation. Although dexmedetomidine was clastogenic in an in vivo mouse micronucleus test in NMRI mice, there was no evidence of clastogenicity in CD-1 mice.
Fertility in male or female rats was not affected after daily subcutaneous injections of dexmedetomidine at doses up to 54 mcg/kg (less than the maximum recommended human intravenous dose on a mcg/m2 basis) administered from 10 weeks prior to mating in males, and 3 weeks prior to mating and during mating in females.
Animal Toxicology and/or Pharmacology
There were no differences in the adrenocorticotropic hormone (ACTH)-stimulated cortisol response in dogs following a single dose of dexmedetomidine compared to saline control. However, after continuous subcutaneous infusions of dexmedetomidine at 3 mcg/kg/hr and 10 mcg/kg/hr for one week in dogs (exposures estimated to be within the clinical range), the ACTH-stimulated cortisol response was diminished by approximately 27% and 40%, respectively, compared to saline-treated control animals indicating a dose-dependent adrenal suppression.
Clinical Studies
The safety and efficacy of dexmedetomidine hydrochloride injection for sedation of non-intubated patients prior to and/or during surgical and other procedures was evaluated in two randomized, double-blind, placebo-controlled multicenter clinical trials. Study 1 evaluated the sedative properties of dexmedetomidine hydrochloride injection in patients having a variety of elective surgeries/procedures performed under monitored anesthesia care. Study 2 evaluated dexmedetomidine hydrochloride injection in patients undergoing awake fiberoptic intubation prior to a surgical or diagnostic procedure.
In Study 1, the sedative properties of dexmedetomidine hydrochloride injection were evaluated by comparing the percent of patients not requiring rescue midazolam to achieve a specified level of sedation using the standardized Observer’s Assessment of Alertness/Sedation Scale (see Table 5).

Patients were randomized to receive a loading infusion of either dexmedetomidine hydrochloride injection 1 mcg/kg, dexmedetomidine hydrochloride injection 0.5 mcg/kg, or placebo (normal saline) given over 10 minutes and followed by a maintenance infusion started at 0.6 mcg/kg/hr. The maintenance infusion of study drug could be titrated from 0.2 mcg/kg/hr to 1 mcg/kg/hr to achieve the targeted sedation score (Observer’s Assessment of Alertness/Sedation Scale ≤ 4). Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain an Observer’s Assessment of Alertness/Sedation Scale ≤ 4. After achieving the desired level of sedation, a local or regional anesthetic block was performed. Demographic characteristics were similar between the dexmedetomidine hydrochloride injection and comparator groups. Efficacy results showed that dexmedetomidine hydrochloride injection was more effective than the comparator group when used to sedate non-intubated patients requiring monitored anesthesia care during surgical and other procedures (see Table 7).
In Study 2, the sedative properties of dexmedetomidine hydrochloride injection were evaluated by comparing the percent of patients requiring rescue midazolam to achieve or maintain a specified level of sedation using the Ramsay Sedation Scale score ≥ 2 (see Table 6).
Patients were randomized to receive a loading infusion of dexmedetomidine hydrochloride injection 1 mcg/kg or placebo (normal saline) given over 10 minutes and followed by a fixed maintenance infusion of 0.7 mcg/kg/hr. After achieving the desired level of sedation, topicalization of the airway occurred. Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain a Ramsay Sedation Scale ≥ 2. Demographic characteristics were similar between the dexmedetomidine hydrochloride injection and comparator groups. For efficacy results see Table 7.

How Supplied
Dexmedetomidine Hydrochloride Injection, Concentrate, 200 mcg/2 mL (100 mcg/mL), for intravenous infusion, is available in:
NDC 67457-251-02 2 mL in a 3 mL single-dose vial, cartons of 25
Storage
Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Medetomidine hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Medetomidine hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Medetomidine hydrochloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Medetomidine hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Medetomidine hydrochloride Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.