Lofexidine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lofexidine is a central alpha-2 adrenergic agonist that is FDA approved for the mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. Common adverse reactions include orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Lofexidine is indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
Dosing Information
- The usual lofexidine starting dosage is three 0.18 mg tablets taken orally 4 times daily during the period of peak withdrawal symptoms (generally the first 5 to 7 days following last use of opioid) with dosing guided by symptoms and side effects. There should be 5 to 6 hours between each dose. The total daily dosage of lofexidine should not exceed 2.88 mg (16 tablets) and no single dose should exceed 0.72 mg (4 tablets).
- Lofexidine treatment may be continued for up to 14 days with dosing guided by symptoms.
- Discontinue lofexidine with a gradual dose reduction over a 2- to 4-day period to mitigate lofexidine withdrawal symptoms (e.g., reducing by 1 tablet per dose every 1 to 2 days). The lofexidine dose should be reduced, held, or discontinued for individuals who demonstrate a greater sensitivity to lofexidine side effects. Lower doses may be appropriate as opioid withdrawal symptoms wane.
- Lofexidine can be administered in the presence or absence of food.
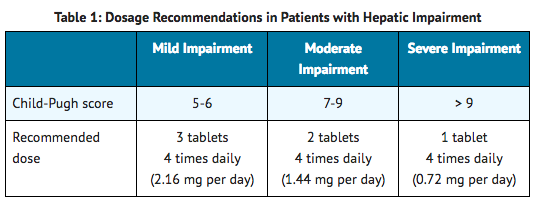
Dosage Recommendations for Patients with Hepatic Impairment
- Recommended dosage adjustments based on the degree of hepatic impairment are shown in Table 1.
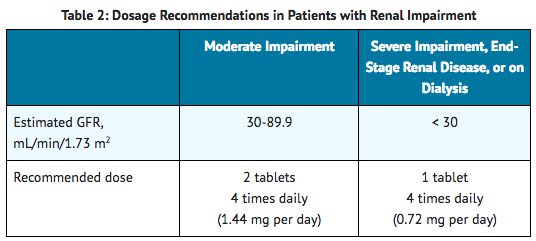
Dosage Recommendations for Patients with Renal Impairment
- Recommended dosage adjustments based on the degree of renal impairment are shown in Table 2. Lofexidine may be administered without regard to the timing of dialysis
Dosage Forms and Strengths
- Lofexidine is available as round, peach-colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side. Each tablet contains 0.18 mg lofexidine (equivalent to 0.2 mg of lofexidine hydrochloride).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding lofexidine Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding lofexidine Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lofexidine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding lofexidine Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding lofexidine Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None.
Warnings
Risk of Hypotension, Bradycardia, and Syncope
- Lofexidine can cause a decrease in blood pressure, a decrease in pulse, and syncope. Monitor vital signs before dosing. Monitor symptoms related to bradycardia and orthostasis.
- Patients being given lofexidine in an outpatient setting should be capable of and instructed on self-monitoring for hypotension, orthostasis, bradycardia, and associated symptoms. If clinically significant or symptomatic hypotension and/or bradycardia occur, the next dose of lofexidine should be reduced in amount, delayed, or skipped.
- Inform patients that lofexidine may cause hypotension and that patients moving from a supine to an upright position may be at increased risk for hypotension and orthostatic effects. Instruct patients to stay hydrated, on how to recognize symptoms of low blood pressure, and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position). Instruct outpatients to withhold lofexidine doses when experiencing symptoms of hypotension or bradycardia and to contact their healthcare provider for guidance on how to adjust dosing.
- Avoid using lofexidine in patients with severe coronary insufficiency, recent myocardial infarction, cerebrovascular disease, chronic renal failure, and in patients with marked bradycardia.
- Avoid using lofexidine in combination with medications that decrease pulse or blood pressure to avoid the risk of excessive bradycardia and hypotension.
Risk of QT Prolongation
- Lofexidine prolongs the QT interval.
- Avoid using lofexidine in patients with congenital long QT syndrome.
- Monitor ECG in patients with congestive heart failure, bradyarrhythmias, hepatic impairment, renal impairment, or patients taking other medicinal products that lead to QT prolongation (e.g., methadone). In patients with electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia), correct these abnormalities first, and monitor ECG upon initiation of lofexidine.
Increased Risk of Central Nervous System Depression with Concomitant use of CNS Depressant Drugs
- Lofexidine potentiates the CNS depressive effects of benzodiazepines and can also be expected to potentiate the CNS depressive effects of alcohol, barbiturates, and other sedating drugs. Advise patients to inform their healthcare provider of other medications they are taking, including alcohol.
- Advise patients using lofexidine in an outpatient setting that, until they learn how they respond to lofexidine, they should be careful or avoid doing activities such as driving or operating heavy machinery.
Increased Risk of Opioid Overdose after Opioid Discontinuation
- Lofexidine is not a treatment for opioid use disorder. Patients who complete opioid discontinuation are likely to have a reduced tolerance to opioids and are at increased risk of fatal overdose should they resume opioid use. Use lofexidine in patients with opioid use disorder only in conjunction with a comprehensive management program for the treatment of opioid use disorder and inform patients and caregivers of this increased risk of overdose.
Risk of Discontinuation Symptoms
- Stopping lofexidine abruptly can cause a marked rise in blood pressure. Symptoms including diarrhea, insomnia, anxiety, chills, hyperhidrosis, and extremity pain have also been observed with lofexidine discontinuation. Instruct patients not to discontinue therapy without consulting their healthcare provider. When discontinuing therapy with lofexidine tablets, gradually reduce the dose.
- Symptoms related to discontinuation can be managed by administration of the previous lofexidine dose and subsequent taper.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to adverse reaction rates observed for another drug and may not reflect the rates observed in practice.
- The safety of lofexidine was supported by three randomized, double-blind, placebo-controlled clinical trials, an open-label study, and clinical pharmacology studies with concomitant administration of either methadone, buprenorphine, or naltrexone.
- The three randomized, double-blind, placebo-controlled clinical trials enrolled 935 subjects dependent on short-acting opioids undergoing abrupt opioid withdrawal. Patients were monitored before each dose in an inpatient setting.
- Table 3 presents the incidence, rounded to the nearest percent, of adverse events that occurred in at least 10% of subjects treated with lofexidine and for which the incidence in patients treated with lofexidine was greater than the incidence in subjects treated with placebo in a study that tested two doses of lofexidine, 2.16 mg per day and 2.88 mg per day, and placebo. The overall safety profile in the combined dataset was similar.
- Orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth were notably more common in subjects treated with lofexidine than subjects treated with placebo.
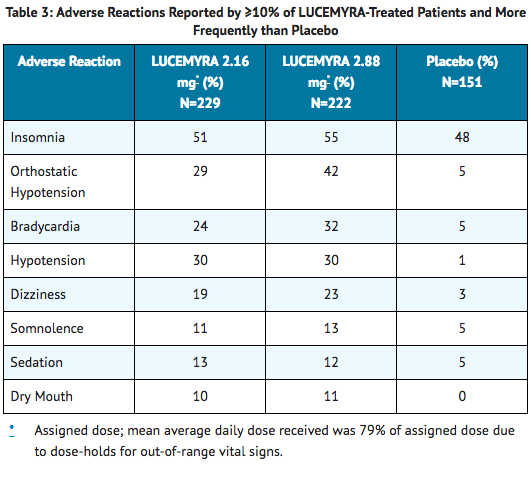
- Other notable adverse reactions associated with the use of lofexidine but reported in <10% of patients in the lofexidine group included:
- Syncope: 0.9%, 1.4% and 0% for lofexidine 2.16 mg/day and 2.88 mg/day and placebo, respectively
- Tinnitus: 0.9%, 3.2% and 0% for lofexidine 2.16 mg/day and 2.88 mg/day and placebo, respectively
Blood pressure changes and adverse reactions after lofexidine cessation
- Elevations in blood pressure above normal values (≥ 140 mmHg systolic) and above a subject's pre-treatment baseline are associated with discontinuing lofexidine, and peaked on the second day after discontinuation, as shown in Table 4. Blood pressure values were evaluated for 3 days following the last dose of a 5-day course of lofexidine 2.88 mg/day.
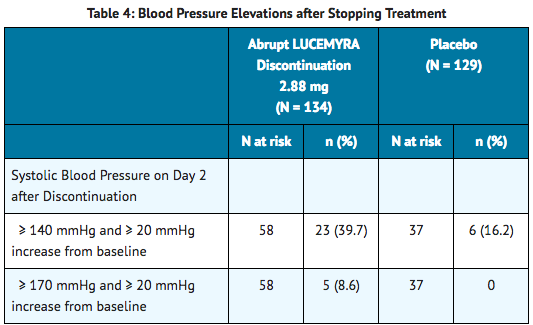
- Blood pressure elevations of a similar magnitude and incidence were observed in a small number of patients (N=10) that had a one-day, 50% dose reduction prior to discontinuation.
- After stopping treatment, subjects that were taking lofexidine also had a higher incidence of diarrhea, insomnia, anxiety, chills, hyperhidrosis, and extremity pain compared to subjects who were taking placebo.
Sex-specific adverse event findings
- Four out of 101 females (4%) had serious cardiovascular adverse events compared to 3 out of 289 (1%) of males assigned to receive lofexidine 2.88 mg per day.
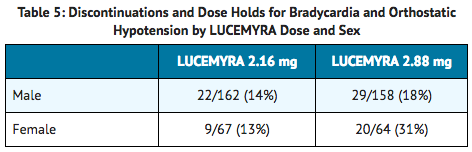
- Discontinuations and dose holds due to bradycardia and orthostatic hypotension, which are the most common adverse reactions associated with lofexidine, occurred with a greater incidence in females assigned to receive the highest studied dose of lofexidine, 2.88 mg per day as shown in Table 5.
Postmarketing Experience
- Lofexidine is marketed in other countries for relief of opioid withdrawal symptoms. The following events have been identified during postmarketing use of lofexidine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Since lofexidine's initial market introduction in 1992, the most frequently reported postmarketing adverse event with lofexidine has been hypotension. There has been one report of QT prolongation, bradycardia, torsades de pointes, and cardiac arrest with successful resuscitation in a patient that received lofexidine and three reports of clinically significant QT prolongation in subjects concurrently receiving methadone with lofexidine.
Drug Interactions
- Methadone
- Oral Naltrexone
- CNS Depressant Drugs
- CYP2D6 Inhibitor - Paroxetine
Methadone
- Lofexidine and methadone both prolong the QT interval. ECG monitoring is recommended in patients receiving methadone and lofexidine.
Oral Naltrexone
- Coadministration of lofexidine and oral naltrexone resulted in statistically significant differences in the steady-state pharmacokinetics of naltrexone. It is possible that oral naltrexone efficacy may be reduced if used concomitantly within 2 hours of lofexidine. This interaction is not expected if naltrexone is administered by non-oral routes.
CNS Depressant Drugs
- Lofexidine potentiates the CNS depressant effects of benzodiazepines and may potentiate the CNS depressant effects of alcohol, barbiturates, and other sedating drugs. Advise patients to inform their healthcare provider of other medications they are taking, including alcohol.
CYP2D6 Inhibitor - Paroxetine
- Coadministration of lofexidine and paroxetine resulted in 28% increase in the extent of absorption of lofexidine. Monitor for orthostatic hypotension and bradycardia when an inhibitor of CYP2D6 is used concomitantly with lofexidine.
Use in Specific Populations
Pregnancy
Risk Summary
- The safety of lofexidine in pregnant women has not been established. In animal reproduction studies, oral administration of lofexidine during organogenesis to pregnant rats and rabbits caused a reduction in fetal weights, increases in fetal resorptions, and litter loss at exposures below that in humans. When oral lofexidine was administered from the beginning of organogenesis through lactation, increased stillbirths and litter loss were noted along with decreased viability and lactation indices. The offspring exhibited delays in sexual maturation, auditory startle, and surface righting. These effects occurred at exposures below that in humans.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies carry some risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects in the U.S. general population is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data (Animal)
- Increased incidence of resorptions, decreased number of implantations, and a concomitant reduction in the number of fetuses were observed when pregnant rabbits were orally administered lofexidine hydrochloride during organogenesis (from gestation day [GD] 7 to 19) at a daily dose of 5.0 mg/kg/day (approximately 0.08 times the maximum recommended human dose [MRHD] of 2.88 mg lofexidine base on an AUC basis). Maternal toxicity evidenced by increased mortality was noted at the highest tested dose of 15 mg/kg/day (approximately 0.4 times the MRHD on an AUC basis).
- Decreased implantations per dam and decreased mean fetal weights were noted in a study in which pregnant rats were treated with oral lofexidine hydrochloride during organogenesis (from GD 7 to 16) at a daily dose of 3.0 mg/kg/day (approximately 0.9 times the MRHD on an AUC basis). This dose was associated with maternal toxicity (decreased body weight gain and mortality). No malformations or evidence of developmental toxicity were evident at 1.0 mg/kg/day (approximately 0.2 times the MRHD on an AUC basis).
- A dose-dependent increase in pup mortality was noted in all doses of lofexidine hydrochloride administered orally to pregnant rats from GD 6 through lactation at an exposure less than the human exposure based on AUC comparisons. Doses higher than 1.0 mg/kg/day (approximately 0.2 times the MRHD on an AUC basis) resulted in incidences of total litter loss and maternal toxicity (piloerection and decreased body weight gain). The highest dose tested of 2.0 mg/kg/day (approximately 0.6 times the MRHD on an AUC basis), increased stillbirths as well as decreased viability and lactation indices were reported. Surviving offspring exhibited lower body weights, developmental delays, and increased delays in auditory startle at doses of 1.0 mg/kg/day or higher. Sexual maturation was delayed in male offspring (preputial separation) at 2.0 mg/kg/day and in female offspring (vaginal opening) at 1.0 mg/kg/day or higher.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lofexidine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lofexidine during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of lofexidine or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Caution should be exercised when lofexidine is administered to a nursing woman.
- The developmental and health benefits should be considered along with the mother's clinical need for lofexidine and any other potential adverse effects on breastfed children from lofexidine or from the underlying maternal condition.
Pediatric Use
- The safety and effectiveness of lofexidine have not been established in pediatric patients.
Geriatic Use
- No studies have been performed to characterize the pharmacokinetics of lofexidine or establish its safety and effectiveness in geriatric patients. Caution should be exercised when it is administered to patients over 65 years of age. Dosing adjustments similar to those recommended in patients with renal impairment should be considered.
Gender
There is no FDA guidance on the use of Lofexidine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lofexidine with respect to specific racial populations.
Renal Impairment
- Renal impairment slows the elimination of lofexidine but exhibits less effect on the peak plasma concentration than on AUC values following a single dose. Dosage adjustments are recommended based on the degree of renal impairment.
- Only a negligible fraction of the lofexidine dose is removed during a typical dialysis session, so no additional dose needs to be administered after a dialysis session; lofexidine may be administered without regard to the timing of dialysis.
- Clinically relevant QT prolongation may occur in subjects with renal impairment.
Hepatic Impairment
- Hepatic impairment slows the elimination of lofexidine but exhibits less effect on the peak plasma concentration than on AUC values following a single dose. Dosage adjustments are recommended based on the degree of hepatic impairment.
- Clinically relevant QT prolongation may occur in subjects with hepatic impairment.
Females of Reproductive Potential and Males
- In animal studies that included some fertility endpoints, lofexidine decreased breeding rate and increased resorptions at exposures below human exposures. The impact of lofexidine on male fertility has not been adequately characterized in animal studies.
Immunocompromised Patients
There is no FDA guidance one the use of Lofexidine in patients who are immunocompromised.
CYP2D6 Poor Metabolizers
- Although the pharmacokinetics of lofexidine have not been systematically evaluated in patients who do not express the drug metabolizing enzyme CYP2D6, it is likely that the exposure to lofexidine would be increased similarly to taking strong CYP2D6 inhibitors (approximately 28%). Monitor adverse events such as orthostatic hypotension and bradycardia in known CYP2D6 poor metabolizers. Approximately 8% of Caucasians and 3–8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM).
Administration and Monitoring
Administration
- Oral.
Monitoring
- Reduction in signs and symptoms of opioid withdrawal and compliance with therapy is indicative of efficacy.
- Vital signs: Prior to dosing.
- Symptoms related to bradycardia and orthostasis: During therapy.
- ECG: In patients with congestive heart failure, bradyarrhythmias, hepatic impairment, renal impairment, or patients taking other medicinal products that lead to QT prolongation (eg, methadone). Correct electrolyte abnormalities prior to ECG monitoring and initiation of therapy.
IV Compatibility
There is limited information regarding the compatibility of Lofexidine and IV administrations.
Overdosage
- Overdose with lofexidine may manifest as hypotension, bradycardia, and sedation. In the event of acute overdose, perform gastric lavage where appropriate. Dialysis will not remove a substantial portion of the drug. Initiate general symptomatic and supportive measures in cases of overdosage.
Pharmacology
| |
Lofexidine
| |
| Systematic (IUPAC) name | |
| (RS)-2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole | |
| Identifiers | |
| CAS number | |
| ATC code | N07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 259.131 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Protein binding | 80–90% |
| Metabolism | Liver (glucuronidation) |
| Half life | 11 hours |
| Excretion | Kidney |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Prescription Only (S4)(AU) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth (tablets) |
Mechanism of Action
- Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic tone.
Structure
Pharmacodynamics
Cardiac Electrophysiology
- Single lofexidine doses of 1.44 to 1.8 mg produced maximum mean change from baseline in QTcF (ΔQTcF) of 14.4 msec (upper two-sided 90% CI: 22.3 msec) and 13.6 msec (17.4 msec) for 1.44 and 1.8 mg respectively in healthy normal volunteers.
- In a Phase 3 placebo-controlled, dose response study in opioid dependent subjects, lofexidine was associated with a maximum mean prolongation of the QTcF interval 7.3 (8.8) and 9.3 (10.9) msec at doses of 2.16 and 2.88 mg/day, respectively.
Patients With Hepatic Impairment
- Administration of lofexidine to subjects with hepatic impairment was associated with prolongation of the QTc interval, which was more pronounced in subjects with severe hepatic impairment.
Patients With Renal Impairment
- Administration of lofexidine to subjects with renal impairment was associated with prolongation of the QTc interval, which was more pronounced in subjects with severe renal impairment.
Lofexidine Coadministered With Methadone
- Lofexidine (2.88 mg/day) coadministered with methadone in 18 methadone-maintained patients (80-120 mg/day) resulted in a maximum mean increase from methadone-alone baseline in QTcF of 9.1 (14.2) msec.
Lofexidine Coadministered With Buprenorphine
- Lofexidine (2.88 mg/day) coadministered with buprenorphine in 21 buprenorphine-maintained patients (16-24 mg/day) resulted in a maximum mean QTcF increase in QTcF of 15 (5.6) msec compared to a buprenorphine-alone baseline.
In Vitro Binding
- Lofexidine exhibits in vitro binding affinity and functional agonist activity with alpha-2A and alpha-2C adrenoreceptors at concentrations within clinical exposure plasma levels (Ki values of approximately 7.2 nM and 12 nM, and EC50 values of 4.9 nM and 0.9 nM, respectively).
Pharmacokinetics
Absorption
- Lofexidine is well absorbed and achieves peak plasma concentration 3 to 5 hours after administration of a single dose.
- Lofexidine shows approximately dose-proportional pharmacokinetics. Administration of lofexidine with food does not alter its pharmacokinetics.
- The absolute bioavailability of a single oral lofexidine dose ( 0.36 mg in solution) compared with an intravenous infusion (0.2 mg infused for 200 minutes) was 72%. Mean lofexidine Cmax after the oral dose and intravenous infusion was 0.82 ng/mL (at median Tmax of 3 hours) and 0.64 ng/mL (at median Tmax of 4 hours), respectively. Mean estimates of overall systemic exposure (AUCinf) were 14.9 ng∙h/mL and 12.0 ng∙h/mL, respectively.
Distribution
- Mean lofexidine apparent volume of distribution and volume of distribution values following the administration of an oral dose and an intravenous dose were 480.0 L and 297.9 L, respectively, which are appreciably greater than total body volume, suggesting extensive lofexidine distribution into body tissue.
- Lofexidine protein binding is approximately 55%.
- Lofexidine is not preferentially taken up by blood cells. In a study comparing lofexidine concentrations in plasma and whole blood at the time of peak lofexidine concentrations in human volunteers, it was determined that red blood cells contain approximately 27% the lofexidine concentration of the plasma.
Elimination
Metabolism
- From absolute bioavailability results, approximately 30% of the administered lofexidine dose is converted to inactive metabolites during the first pass effect associated with drug absorption from the gut.
- Lofexidine and its major metabolites did not induce or inhibit any CYP450 isoforms, with the exception of a slight inhibition of CYP2D6 by lofexidine, with an IC50 of 4551 nM (approximately 225 times the steady-state Cmax for lofexidine with 0.72 mg 4 times daily dosing). Any lofexidine interaction with CYP2D6 substrates is not expected to be clinically significant.
- Lofexidine is metabolized when incubated in vitro with human liver microsomes, the major contributor to the hepatic metabolism of lofexidine is CYP2D6, with CYP1A2 and CYP2C19 also capable of metabolizing lofexidine.
Excretion
- The elimination half-life is approximately 12 hours and mean clearance is 17.6 L/h following an IV infusion.
- Lofexidine has a terminal half-life of approximately 11 to 13 hours following the first dose. At steady-state, the terminal half-life is approximately 17 to 22 hours. Accumulation occurs up to 4 days with repeat dosing, following the recommended dosing regimen.
- A mass balance study of lofexidine showed nearly complete recovery of radiolabel in urine (93.5%) over 144 hours postdose, with an additional 0.92% recovered in the feces over 216 hours postdose. Thus, it appears that all, or nearly all, of the dose was absorbed, and that the primary route of elimination of the parent drug and its metabolites is via the kidney. Renal elimination of unchanged drug accounts for approximately 15% to 20% of the administered dose.
Specific Populations
Hepatic Impairment
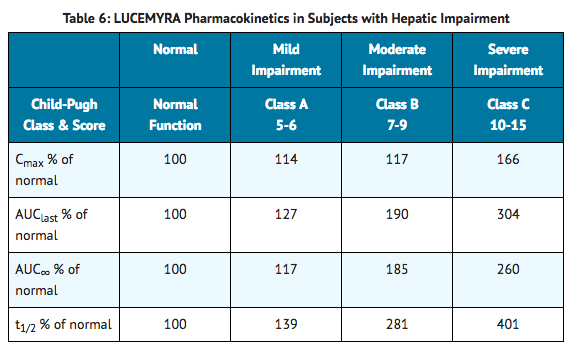
- Hepatic impairment slows the elimination of lofexidine, but exhibits less effect on the peak plasma concentration following a single dose. In a study comparing the pharmacokinetics of lofexidine (0.36 mg) in mild, moderate, and severe hepatically impaired subjects to subjects with normal hepatic function (6 subjects in each hepatic function group), mean Cmax values were similar for subjects with normal, mild, and moderate hepatic impairment as shown in Table 6.
Renal Impairment
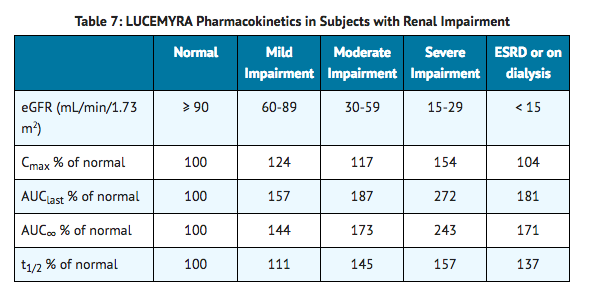
- Renal impairment slows the elimination of lofexidine but exhibits less effect on the peak plasma concentration following a single dose. In a study comparing the pharmacokinetics of lofexidine (0.36 mg) in 8 end-stage renal disease subjects on 3 times weekly hemodialysis to 8 subjects with normal renal function matched for sex, age, and body mass index, mean Cmax values were similar for end-stage renal disease and normal renal function subjects, indicating no change in maximum lofexidine exposure with renal impairment as shown in Table 7.
- The impact of dialysis on the overall pharmacokinetics of lofexidine during a typical 4-hour dialysis was minimal; the drop in lofexidine plasma concentrations produced during the dialysis session was transient, with a rebound to nearly predialysis concentrations after re-equilibration within a few hours following completion of the dialysis cycle.
- In a study comparing the pharmacokinetics of lofexidine (0.36 mg) in 6 subjects each with normal renal function, mild renal impairment, and moderate renal impairment as well as 5 subjects with severe renal impairment but not requiring dialysis, there were similar increases in mean Cmax values in subjects with mild and moderate renal impairment in comparison to subjects with normal renal function with additional increase in mean Cmax values in subjects with severe renal impairment. Mean AUClast, AUC∞, and t1/2 increased with severity of renal impairment as shown in Table 7.
Drug Interaction Studies
Lofexidine coadministered with methadone
- In a double-blind placebo-controlled study of 23 patients maintained on a methadone dose of 80-120 mg/day and concomitantly administered lofexidine up to 2.88 mg/day, lofexidine did not alter the pharmacokinetics of methadone. Lofexidine concentrations may be slightly increased when coadministered with methadone; however, the increase at concentrations expected with recommended dosing is not clinically meaningful.
Lofexidine coadministered with buprenorphine
- In a double-blind placebo-controlled study of 30 subjects maintained on buprenorphine (16-24 mg/day) concomitantly administered lofexidine up to 2.88 mg/day, no pharmacokinetic or pharmacodynamic interactions between lofexidine and buprenorphine were seen.
Lofexidine coadministered with oral naltrexone
- In an open-label, single-arm study of 24 healthy subjects, oral naltrexone (50 mg/day) did not significantly alter the single-dose pharmacokinetics of lofexidine (0.36 mg). The alteration in steady-state pharmacokinetics of oral naltrexone was statistically significant in the presence of lofexidine. The tmax was delayed for both naltrexone and 6ß-naltrexol (2-3 hours), and overall exposure was slightly reduced when naltrexone was administered with lofexidine.
Lofexidine coadministered with paroxetine
- In an open-label, single-sequence study of 24 healthy subjects, the strong CYP2D6 inhibitor paroxetine (40 mg/day) increased lofexidine (0.36 mg) Cmax and AUC∞ by approximately 11% and 28%, respectively.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- No adequate long-term animal studies have been completed to evaluate the carcinogenic potential of lofexidine.
Mutagenesis
- Lofexidine tested positive in the in vitro mouse lymphoma assay. Lofexidine tested negative in the in vitro bacterial reverse mutation assay (Ames assay) and in the in vivo rat micronucleus assay.
Impairment of Fertility
- In a female fertility study in rabbits, fertility was not adversely impacted by administration of lofexidine hydrochloride up to 6.4 mg/kg/day (approximately 0.1 times the MRHD of 2.88 mg on an AUC basis) when administered orally to female rabbits starting 2 weeks prior to mating and through gestation and lactation. However, decreased breeding rate and higher post-implantation loss was observed at this dose, which correlated with higher resorptions and reduced litter size. Maternal toxicity, which included increased mortality rate, reduced body weight gain, and moderate sedation was observed at 6.4 mg/kg/day. The NOAEL for female fertility was 6.4 mg/kg/day and the NOAEL for female-mediated developmental parameters was 0.4 mg/kg/day (approximately 0.005 times the MRHD on an AUC basis).
- In a fertility study in rats, fertility was unaffected by administration of lofexidine up to 0.88 mg/kg/day (approximately 0.2 times the MRHD on an AUC basis) via diet to male and female rats prior to mating and to the dams through gestation and lactation. No evidence of maternal toxicity was observed. However, no assessment of sperm or reproductive organs were performed in this study.
- Reduced testes, epididymis, and seminiferous tubule weights, as well as delayed sexual maturation of males and females and decreases in the number of corpora lutea and implantations after mating, were noted in offspring of pregnant rats administered lofexidine hydrochloride orally from GD 6 through lactation at exposures less than the human exposure based on AUC comparisons.
Clinical Studies
- Two randomized, double-blind, placebo-controlled trials supported the efficacy of lofexidine.
Study 1, NCT01863186
- Study 1 was a 2-part efficacy, safety, and dose-response study conducted in the United States in patients meeting DSM-IV criteria for opioid dependence who were physically dependent on short-acting opioids (e.g., heroin, hydrocodone, oxycodone). The first part of the study was an inpatient, randomized, double-blind, placebo-controlled design consisting of 7 days of inpatient treatment (Days 1 – 7) with lofexidine 2.16 mg total daily dose (0.54 mg 4 times daily) (n=229), lofexidine 2.88 mg total daily dose (0.72 mg 4 times daily) (n=222), or matching placebo (n=151). Patients also had access to a variety of support medications for withdrawal symptoms (guaifenesin, antacids, dioctyl sodium sulfosuccinate, psyllium hydrocolloid suspension, bismuth sulfate, acetaminophen, and zolpidem). The second part of the study (Days 8 – 14) was an open-label design where all patients who successfully completed Days 1 – 7 were eligible to receive open-label treatment with variable dose lofexidine treatment (as determined by the investigator, but not to exceed 2.88 mg total daily dose) for up to an additional 7 days (Days 8 – 14) in either an inpatient or outpatient setting as determined by the investigator and the patient. No patient received lofexidine for more than 14 days.
- The two endpoints to support efficacy were the mean Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop) total score on Days 1 – 7 of treatment and the proportion of patients that completed 7 days of treatment. The SOWS-Gossop, a patient-reported outcome (PRO) instrument, evaluates the following opioid withdrawal symptoms: feeling sick, stomach cramps, muscle spasms/twitching, feeling of coldness, heart pounding, muscular tension, aches and pains, yawning, runny eyes and insomnia/problems sleeping. For each opioid withdrawal symptom, patients are asked to rate their symptom severity using four response options (none, mild, moderate, and severe). The SOWS-Gossop total score ranges from 0 to 30 where a higher score indicates a greater withdrawal symptom severity. The SOWS-Gossop was administered at baseline and once daily 3.5 hours after the first morning dose on Days 1 – 7.
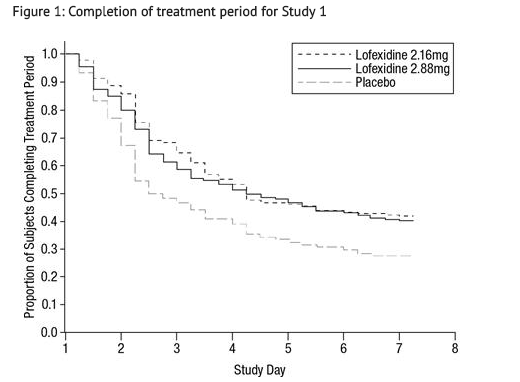
- Of the randomized and treated patients, 28% of placebo patients, 41% of lofexidine 2.16 mg and 40% of lofexidine 2.88 mg patients completed 7 days of treatment. The difference in proportion in both lofexidine groups was significant compared to placebo. See FIGURE 1. Patients in the placebo group were more likely to drop out of the study prematurely due to lack of efficacy than patients treated with lofexidine.
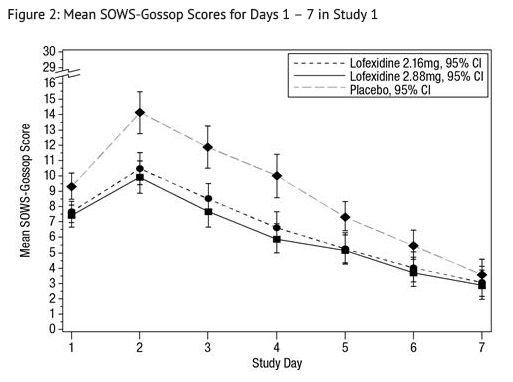
- The mean SOWS-Gossop scores for Days 1 – 7 were 8.8, 6.5, and 6.1 for placebo, lofexidine 2.16 mg and lofexidine 2.88 mg, respectively. Results are shown in Figure 2. The mean difference between lofexidine 2.16 mg and placebo was -2.3 with a 95% CI of (-3.4, -1.2). The mean difference between lofexidine 2.88 mg and placebo was -2.7 with a 95% CI of (-3.9, -1.6). They were both significant. Symptoms assessed on the SOWS-Gossop were recorded as absent or mild for almost all patients remaining to the end of the assessment period.
Study 2, NCT00235729
- Study 2 was an inpatient, randomized, multicenter, double-blind, placebo-controlled study carried out in the United States in patients meeting DSM-IV criteria for opioid dependence who were physically dependent on short-acting opioids (e.g., heroin, hydrocodone, oxycodone). Patients were treated with lofexidine tablets (2.88 mg/day [0.72 mg four times daily]) or matching placebo for 5 days (Days 1 – 5). Patients also had access to a variety of support medications for withdrawal symptoms (guaifenesin, antacids, dioctyl sodium sulfosuccinate, psyllium hydrocolloid suspension, bismuth sulfate, acetaminophen, and zolpidem). All patients then received placebo on Days 6 and 7 and were discharged on Day 8.
- The two endpoints to support efficacy were the mean SOWS-Gossop total score on Days 1 – 5 of treatment and the proportion of patients that completed 5 days of treatment. The SOWS-Gossop was administered at baseline and once daily 3.5 hours after the first morning dose on Days 1 – 5.
- A total of 264 patients were randomized into the study. Of these, 134 patients were randomized to lofexidine 2.88 mg/day and 130 patients to placebo.
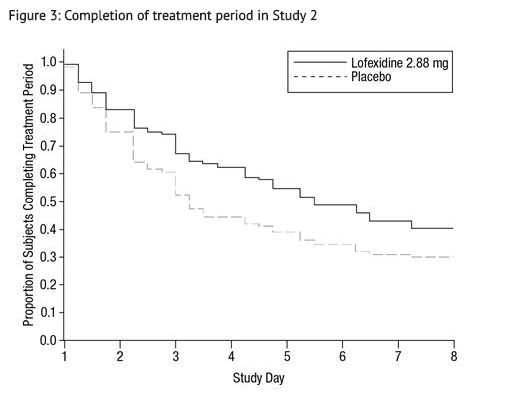
- Of the randomized and treated patients, 33% of placebo patients and 49% of lofexidine patients completed 5 days of treatment. The difference in proportion between the two groups was significant. See FIGURE 3. Patients in the placebo group were more likely to drop out of the study prematurely due to lack of efficacy than patients treated with lofexidine.
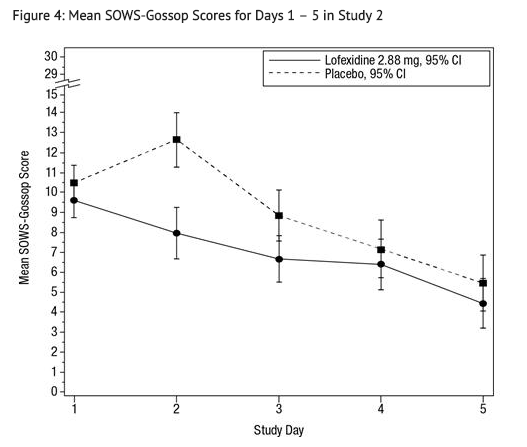
- The mean SOWS-Gossop scores for Days 1 – 5 were 8.9 and 7.0 for placebo and lofexidine 2.88 mg, respectively. Results are shown in Figure 4. The mean difference was -1.9 with a 95% CI of (-3.2, -0.6) and was statistically significant.
How Supplied
- Available as 0.18 mg round, convex-shaped, peach colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side; approximately 7 mm in diameter.
- Bottles of 36 tablets NDC 27505-050-36
- Bottles of 96 tablets NDC 27505-050-96
Storage
- Store in original container at controlled room temperature, 25°C (77°F); with excursions permitted between 15°C to 30°C (59°F to 86°F). Keep lofexidine away from excess heat and moisture both in the pharmacy and after dispensing. Do not remove desiccant packs from bottles until all tablets are used. Keep lofexidine and all medicines out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Lofexidine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lofexidine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients to read the FDA-approved patient labeling.
- Lofexidine may mitigate, but not completely prevent, the symptoms associated with opioid withdrawal syndrome, which may include feeling sick, stomach cramps, muscle spasms or twitching, feeling of cold, heart pounding, muscular tension, aches and pains, yawning, runny eyes and sleep problems (insomnia). Patients should be advised that withdrawal will not be easy. Additional supportive measures should be clearly advised, as needed.
Hypotension and Bradycardia
- Inform patients to be alert for any symptoms of low blood pressure or pulse (e.g., dizziness, lightheadedness, or feelings of faintness at rest or on abruptly standing). Advise patients on how to reduce the risk of serious consequences should hypotension occur (sit or lie down, carefully rise from a sitting or lying position).
- Patients being given lofexidine in an outpatient setting should be capable of and instructed on self-monitoring for hypotension, orthostasis and bradycardia and advised to withhold lofexidine doses and contact their healthcare provider for instructions if they experience these signs or related symptoms.
- Advise patients to avoid becoming dehydrated or overheated, which may potentially increase the risks of hypotension and syncope.
Concomitant Medications
- Review with patients all concomitant medications being taken and request that they immediately inform their healthcare provider of any changes in concomitant medications, including any other medications that may be used to treat individual symptoms of withdrawal.
Increased Risk of CNS Depression with Concomitant use of CNS Depressant Drugs
- Inform patients of the increased risk of CNS depression with concomitant use of benzodiazepines, alcohol, barbiturates, or other sedating drugs.
- Advise patients using lofexidine in an outpatient setting that, until they learn how they respond to lofexidine, they should be careful or avoid doing activities such as driving or operating heavy machinery.
Sudden Discontinuation of Lofexidine
- Sudden Discontinuation of lofexidine.
Risk of Opioid Overdose After Discontinuation of Opioids
- Advise patients that after a period of not using opioid drugs, they may be more sensitive to the effects of opioids and at greater risk of overdosing.

Precautions with Alcohol
Alcohol-Lofexidine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Lucemyra
Look-Alike Drug Names
There is limited information regarding Lofexidine Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.