Baclofen
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Baclofen is a skeletal muscle relaxant, spasmolytic that is FDA approved for the treatment of spasticity. Common adverse reactions include cardiovascular: hypotension, gastrointestinal: constipation, nausea, vomiting, musculoskeletal: Poor muscle tone, neurologic: asthenia, dizziness, urinary complication, fatigue, and shivering.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Do not abruptly discontinue or withdraw therapy.
- Signs of overdose may appear suddenly and insidiously.
Spasticity
- Oral
- 5 mg orally 3 times a day; may increase dosage by 15 mg/day increments every 3 days to a max dose of 80 mg/day (3 to 4 divided doses)
- Intrathecal
- Test dose, 50 mcg in 1 mL intrathecally given over at least 1 minute; may increase dosage by 25 mcg increments every 24 hours until a 4- to 8-hour positive clinical response is demonstrated. Patients must respond to a single bolus dose of no greater than 100 mcg/2mL to be acceptable candidates for chronic therapy with the intrathecal infusion pump.
- Post-implant titration, initial (test dose efficacy less than 8 hours), the initial daily dose is double the screening dose administered intrathecally over 24 hour.
- Post-implant titration, initial (test dose efficacy greater than 8 hour), the initial daily dose is the same as the screening dose administered intrathecally over 24 hours.
- Post-implant titration, spinal cord spasticity, no dosage increases during first 24 hours; may slowly increase intrathecal dosage by 10% to 30% once every 24 hours.
- Post-implant titration, cerebral origin spasticity, no dosage increases during the first 24 hours; may slowly increase intrathecal dosage by 5% to 15% once every 24 hours.
- Post-implant maintenance, spinal cord spasticity, intrathecal daily dose may be increased by 10% to 40% (max 40%) OR reduced by 10% to 20% as needed during periodic pump refills. Most patients require gradual increase in dose over time to maintain optimal response. Maintenance dosages usually range between 300 to 800 mcg/day (range 12 to 2003 mcg/day).
- Post-implant maintenance, cerebral origin spasticity, intrathecal daily dose may be increased by 5% to 20% (max 20%) OR reduced by 10% to 20% as needed during periodic pump refills. Most patients require gradual increase in dose over time to maintain optimal response. Maintenance dosages usually range between 90 to 703 mcg/day (range 22 to 1400 mcg/day).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Intractable hiccough
- Spasm of bladder.
- Stiff-man syndrome.
- Tetanus.
- Trigeminal neuralgia.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Baclofen in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of oral baclofen use in children under 12 years of age have not been established.
- Safety and effectiveness have not been established in children under 4 years of age.
- Do not abruptly discontinue or withdraw therapy.
- Signs of overdose may appear suddenly and insidiously.
Spasticity
- Oral
- 2 to 7 years old
- 10 to 15 mg/day orally (2 to 3 divided doses); may increase by 5 to 15 mg/day increments every 3 days to a max dose of 40 mg/day (3 to 4 divided doses).
- 8 years and older
- 10 to 15 mg/day orally (2 to 3 divided doses); may increase by 5 to 15 mg/day increments every 3 days to a MAX dose of 60 mg/day (3 to 4 divided doses).
- Intrathecal
- Test dose, 25 to 50 mcg intrathecally given over at least 1 minute; may increase dosage by 25 mcg increments every 24 hour until a 4- to 8-hour positive clinical response is demonstrated. Patients must respond to a single bolus dose of no greater than 100 mcg/2mL to be acceptable candidates for chronic therapy with the intrathecal infusion pump.
- Post-implant titration, after the first 24 hour, the intrathecal daily dose should be increased slowly by 5% to 15% only once every 24 hour, until the desired clinical effect is achieved.
- Post-implant maintenance, intrathecal daily dose may be increased by 5% to 20% (MAX 20%) OR reduced by 10% to 20% as needed during periodic pump refills. Most patients require gradual increase in dose over time to maintain optimal response. The average maintenance dose for children under age 12 years is 274 mcg/day (range 24 to 1199 mcg/day).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Baclofen in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Baclofen in pediatric patients.
Contraindications
- Hypersensitivity to baclofen.
Warnings
Abrupt Drug Withdrawal
- Hallucinations and seizures have occurred on abrupt withdrawal of baclofen. Therefore, except for serious adverse reactions, the dose should be reduced slowly when the drug is discontinued.
Impaired Renal Function
- Because baclofen is primarily excreted unchanged through the kidneys, it should be given with caution, and it may be necessary to reduce the dosage.
Stroke
- Baclofen has not significantly benefited patients with stroke. These patients have also shown poor tolerability to the drug.
Pregnancy
- Baclofen has been shown to increase the incidence of omphaloceles (ventral hernias) in fetuses of rats given approximately 13 times the maximum dose recommended for human use, at a dose which caused significant reductions in food intake and weight gain in dams. This abnormality was not seen in mice or rabbits.
- There was also an increased incidence of incomplete sternebral ossification in fetuses of rats given approximately 13 times the maximum recommended human dose, and an increased incidence of unossified phalangeal nuclei of forelimbs and hindlimbs in fetuses of rabbits given approximately 7 times the maximum recommended human dose. In mice, no teratogenic effects were observed, although reductions in mean fetal weight with consequent delays in skeletal ossification were present when dams were given 17 and 34 times the human daily dose. There are no studies in pregnant women. Baclofen should be used during pregnancy only if the benefit clearly justifies the potential risk to the fetus.
Adverse Reactions
Clinical Trials Experience
- The most common is transient drowsiness (10-63%). In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the placebo group. Other common adverse reactions are dizziness (5-15%), weakness (5-15%) and fatigue (2-4%)
Others reported
Neurospsychiatric
- Confusion (1-11%), headache (4-8%), insomnia (2-7%); and rarely, euphoria, excitement, depression, hallucinations, paresthesia, muscle pain, tinnitus, slurred speech, coordination disorder, tremor, rigidity, dystonia, ataxia, blurred vision, nystagmus, strabismus, miosis, mydriasis, diplopia, dysarthria, epileptic seizure.
Cardiovascular
- Hypotension (0-9%). Rare instances of dyspnea, palpitation, chest pain, syncope.
Gastrointestinal
- Nausea (4-12%), constipation (2-6%); and rarely, dry mouth, anorexia, taste disorder, abdominal pain, vomiting, diarrhea, and positive test for occult blood in stool.
Genitourinary
- Urinary frequency (2-6%); and rarely, enuresis, urinary retention, dysuria, impotence, inability to ejaculate, nocturia, hematuria.
Other
- Instances of rash, pruritis, ankle edema, excessive perspiration, weight gain, nasal congestion. Some of the CNS and genitourinary symptoms may be related to the underlying disease rather than to drug therapy. The following laboratory tests have been found to be abnormal in a few patients receiving baclofen; increased SGOT, elevated alkaline phosphatase, and elevation of blood sugar.
Postmarketing Experience
There is limited information regarding Baclofen Postmarketing Experience in the drug label.
Drug Interactions
- There is limited information regarding drug interactions of Baclofen.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Baclofen in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Baclofen in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Baclofen during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Baclofen in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Baclofen in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Baclofen in geriatric settings.
Gender
There is no FDA guidance on the use of Baclofen with respect to specific gender populations.
Race
There is no FDA guidance on the use of Baclofen with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Baclofen in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Baclofen in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Baclofen in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Baclofen in patients who are immunocompromised.
Administration and Monitoring
Administration
The determination of optimal dosage requires individual titration. Start therapy at a low dosage and increase gradually until optimum effect is achieved (usually between 40-80 mg daily).
- The following dosage titration schedule is suggested:
- 5 mg t.i.d. for 3 days
- 10 mg t.i.d. for 3 days
- 15 mg t.i.d. for 3 days
- 20 mg t.i.d. for 3 days
- Thereafter additional increases may be necessary but the total dose should not exceed a maximum of 80 mg daily (20 mg q.i.d).
- The lowest dose compatible with an optimal response is recommended. If benefits are not evident after a reasonable trial period, patients should be slowly withdrawn from the drug (see Warnings Abrupt Drug Withdrawal).
Monitoring
There is limited information regarding Baclofen Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Baclofen and IV administrations.
Overdosage
Signs and Symptoms
- Vomiting, muscular hypotonia, drowsiness, accommodation disorders, coma, respiratory depression and seizures.
Treatment
- In the alert patient, empty the stomach promptly by induced emesis followed by lavage. In the obtunded patient, secure the airway with a cuffed endotracheal tube before beginning lavage (do not induce emesis). Maintain adequate respiratory exchange, do not use respiratory stimulants.
Pharmacology
Mechanism of Action
- The precise mechanism of action of baclofen is not fully known. Baclofen is capable of inhibiting both monosynaptic and polysynaptic reflexes at the spinal level, possibly by hyperpolarization of afferent terminals, although actions at supraspinal sites may also occur and contribute to its clinical effect. Although baclofen is an analog of the putative inhibitory neurotransmitter gamma-aminobutyric acid (GABA), there is no conclusive evidence that actions on GABA systems are involved in the production of its clinical effects. In studies with animals baclofen has been shown to have general CNS depressant properties as indicated by the production of sedation with tolerance, somnolence, ataxia, and respiratory and cardiovascular depression.
Structure
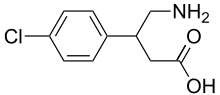
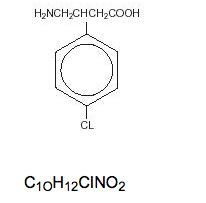
- Its chemical name is 4-amino-3-(4-chlorophenyl) butanoic acid. The structural formula is:
Pharmacodynamics
There is limited information regarding Baclofen Pharmacodynamics in the drug label.
Pharmacokinetics
- Baclofen is rapidly and extensively absorbed and eliminated. Absorption may be dose-dependent, being reduced with increasing doses. Baclofen is excreted primarily by the kidney in unchanged form and there is relatively large inter-subject variation in absorption and/or elimination.
Nonclinical Toxicology
There is limited information regarding Baclofen Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Baclofen Clinical Studies in the drug label.
How Supplied
- Baclofen Tablets, USP, 10 mg are white to off white, round, flat face bevel edge, uncoated tablets debossed ‘291’ on one side and scored on other side are available as follows:
- Bottles of 100 NDC 57664-291-88
- Bottles of 500 NDC 57664-291-13
- Bottles of 1000 NDC 57664-291-18
- Baclofen Tablets, USP, 20 mg are white to off white, round, flat face bevel edge, uncoated tablets debossed ‘292’ on one side and scored on other side are available as follows:
- Bottles of 100 NDC 57664-292-88
- Bottles of 500 NDC 57664-292-13
- Bottles of 1000 NDC 57664-292-18
- Pharmacist: Dispense in well closed container with child resistant closure as defined in USP.
Storage
- Store at 20 - 25° C (68 - 77° F) [See USP Controlled Room Temperature].
C.S. No.: 5532T01
Images
Drug Images
{{#ask: Page Name::Baclofen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Baclofen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Baclofen Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Baclofen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Baclofen Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Baclofen Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Baclofen |Pill Name=Bacolfen_NDC_05915730.jpg |Drug Name=Bacolfen |Pill Ingred=Baclofen[baclofen]|+sep=; |Pill Imprint=DAN;5730;10 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05915730
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Bacolfen_NDC_05915731.jpg |Drug Name=Bacolfen |Pill Ingred=Baclofen[baclofen]|+sep=; |Pill Imprint=DAN;5731;20 |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05915731
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_09043365.jpg |Drug Name=Baclofen |Pill Ingred=BACLOFEN[BACLOFEN]|+sep=; |Pill Imprint=4096;10 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09043365
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_576640291.jpg |Drug Name=Baclofen |Pill Ingred=Baclofen[Baclofen]|+sep=; |Pill Imprint=291 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=2 |Pill Image= |Drug Author=CARACO PHARMACEUTICAL LABORATORIES, LTD. |NDC=576640291
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_06032406.jpg |Drug Name=Baclofen |Pill Ingred=BACLOFEN[BACLOFEN]|+sep=; |Pill Imprint=2265;V |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032406
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_06032407.jpg |Drug Name=Baclofen |Pill Ingred=BACLOFEN[BACLOFEN]|+sep=; |Pill Imprint=2266;V |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=2 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032407
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_08321024.jpg |Drug Name=Baclofen |Pill Ingred=Baclofen[Baclofen]|+sep=; |Pill Imprint=BAC;10;832 |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=Upsher-Smith Laboratories, Inc. |NDC=08321024
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_08321025.jpg |Drug Name=Baclofen |Pill Ingred=Baclofen[Baclofen]|+sep=; |Pill Imprint=832;BC20 |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Upsher-Smith Laboratories, Inc. |NDC=08321025
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_01724096.jpg |Drug Name=Baclofen |Pill Ingred=BACLOFEN[BACLOFEN]|+sep=; |Pill Imprint=4096;TV |Pill Dosage=10 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=IVAX Pharmaceuticals, Inc. |NDC=01724096
}}
{{#subobject:
|Page Name=Baclofen |Pill Name=Baclofen_NDC_01724097.jpg |Drug Name=Baclofen |Pill Ingred=BACLOFEN[BACLOFEN]|+sep=; |Pill Imprint=4097;TV |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=IVAX Pharmaceuticals, Inc. |NDC=01724097
}}
{{#subobject:
|Label Page=Baclofen |Label Name=Baclofen label.png
}}