Levothyroxine (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
* NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS Thyroid hormones, including Levothyroxine Sodium for Injection , should not be used for the treatment of obesity or for weight loss.
|
Overview
Levothyroxine (injection) is a thyroid Supplement that is FDA approved for the treatment of myxedema coma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include weight loss, increased appetite, palpitations, nervousness, diarrhea, abdominal cramps, sweating, tachycardia, insomnia and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Levothyroxine Sodium for Injection is indicated for the treatment of myxedema coma. Important Limitations of Use: The relative bioavailability between Levothyroxine Sodium for Injection and oral levothyroxine products has not been established. Caution should be used when switching patients from oral levothyroxine products to Levothyroxine Sodium for Injection as accurate dosing conversion has not been studied.
Dosage
- An initial intravenous loading dose of Levothyroxine Sodium for Injection between 300 to 500 mcg, followed by once daily intravenous maintenance doses between 50 and 100 mcg, should be administered, as clinically indicated, until the patient can tolerate oral therapy. The age, general physical condition, cardiac risk factors, and clinical severity of myxedema and duration of myxedema symptoms should be considered when determining the starting and maintenance dosages of Levothyroxine Sodium for Injection.
- Levothyroxine Sodium for Injection produces a gradual increase in the circulating concentrations of the hormone with an approximate half-life of 9 to 10 days in hypothyroid patients. Daily administration of Levothyroxine Sodium for Injection should be maintained until the patient is capable of tolerating an oral dose and is clinically stable. For chronic treatment of hypothyroidism, an oral dosage form of levothyroxine should be used to maintain a euthyroid state. Relative bioavailability between Levothyroxine Sodium for Injection and oral levothyroxine products has not been established. Based on medical practice, the relative bioavailability between oral and intravenous administration of Levothyroxine Sodium for Injection is estimated to be from 48 to 74%. Due to differences in absorption characteristics of patients and the oral levothyroxine product formulations, TSH and thyroid hormone levels should be measured a few weeks after initiating oral levothyroxine and dose adjusted accordingly.
Dosing in the Elderly and in Patients with Cardiovascular Disease
- Intravenous levothyroxine may be associated with cardiac toxicity—including arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death—in the elderly and in those with underlying cardiovascular disease. Therefore, cautious use, including doses in the lower end of the recommended range, may be warranted in these populations.
Reconstitution Directions
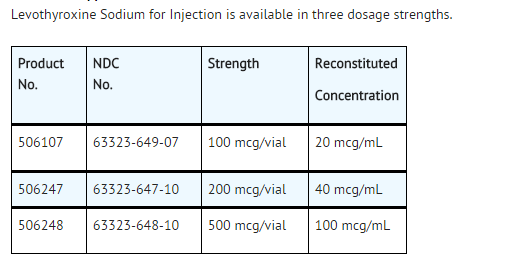
- Reconstitute the lyophilized Levothyroxine Sodium for Injection by aseptically adding 5 mL of 0.9% Sodium Chloride Injection, USP only. Shake vial to ensure complete mixing. The resultant solution will have a final concentration of approximately 20 mcg per mL, 40 mcg per mL and and 100 mcg per mL for the 100 mcg, 200 mcg and 500 mcg vials, respectively. Reconstituted drug product is preservative free and is stable for 4 hours. Discard any unused portion. DO NOT ADD LEVOTHYROXINE SODIUM FOR INJECTION TO OTHER IV FLUIDS. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
DOSAGE FORMS AND STRENGTHS
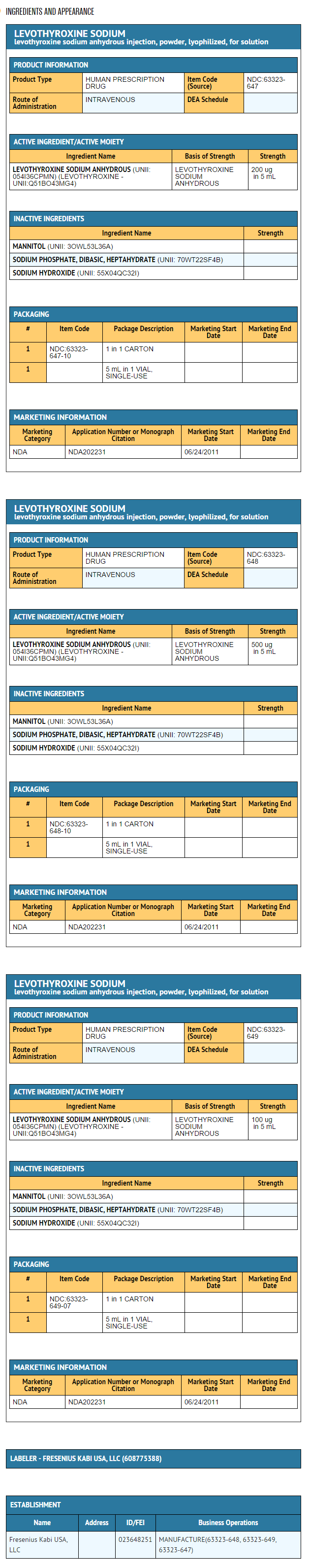
- Levothyroxine Sodium for Injection is supplied as a lyophilized powder at three strengths in single use amber-colored vials: 100 mcg, 200 mcg and 500 mcg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levothyroxine (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levothyroxine (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Levothyroxine (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levothyroxine (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levothyroxine (injection) in pediatric patients.
Contraindications
- None
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
* NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS Thyroid hormones, including Levothyroxine Sodium for Injection , should not be used for the treatment of obesity or for weight loss.
|
Risk of Cardiac Complications in Elderly and in Patients with Cardiovascular Disease
- Excessive bolus dosing of Levothyroxine Sodium for Injection (greater than 500 mcg) are associated with cardiac complications, particularly in the elderly and in patients with an underlying cardiac condition. Adverse events that can potentially be related to the administration of large doses of Levothyroxine Sodium for Injection include arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death. Cautious use, including doses in the lower end of the recommended range, may be warranted in these populations. Close observation of the patient following the administration of Levothyroxine Sodium for Injection is advised.
Need for Concomitant Glucocorticoids and Monitoring for Other Diseases in Patients with Endocrine Disorders
- Occasionally, chronic autoimmune thyroiditis, which can lead to myxedema coma, may occur in association with other autoimmune disorders such as adrenal insufficiency, pernicious anemia, and insulin‑dependent diabetes mellitus. Patients should be treated with replacement glucocorticoids prior to initiation of treatment with Levothyroxine Sodium for Injection, until adrenal function has been adequately assessed. Failure to do so may precipitate an acute adrenal crisis when thyroid hormone therapy is initiated, due to increased metabolic clearance of glucocorticoids by thyroid hormone. With initiation of Levothyroxine Sodium for Injection, patients with myxedema coma should also be monitored for previously undiagnosed diabetes insipidus.
Not Indicated for Treatment of Obesity
- Thyroid hormones, including Levothyroxine Sodium for Injection, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects
Adverse Reactions
Clinical Trials Experience
- Excessive doses of levothyroxine can predispose to signs and symptoms compatible with hyperthyroidism. The signs and symptoms of thyrotoxicosis include, but are not limited to: exophthalmic goiter, weight loss, increased appetite, palpitations, nervousness, diarrhea, abdominal cramps, sweating, tachycardia, increased pulse and blood pressure, cardiac arrhythmias, angina pectoris, tremors, insomnia, heat intolerance, fever, and menstrual irregularities.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Levothyroxine (injection) in the drug label.
Drug Interactions
- Many drugs affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to Levothyroxine Sodium for Injection. In addition, thyroid hormones and thyroid status have varied effects on the pharmacokinetics and actions of other drugs.
Antidiabetic Therapy
- Addition of levothyroxine to antidiabetic or insulin therapy may result in increased antidiabetic agent or insulin requirements. Careful monitoring of diabetic control is recommended, especially when thyroid therapy is started, changed, or discontinued.
Oral Anticoagulants
- Levothyroxine increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the Levothyroxine Sodium for Injection dose is increased. Prothrombin time should be closely monitored to permit appropriate and timely dosage adjustments.
Digitalis Glycosides
- The therapeutic effects of digitalis glycosides may be reduced by levothyroxine. Serum digitalis glycoside levels may be decreased when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
Antidepressant Therapy
- Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and levothyroxine may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and CNS stimulation; onset of action of tricyclics may be accelerated. Administration of sertraline in patients stabilized on levothyroxine may result in increased levothyroxine requirements.
Ketamine
- Concurrent use may produce marked hypertension and tachycardia; cautious administration to patients receiving thyroid hormone therapy is recommended.
Sympathomimetics
- Concurrent use may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
Drug-Laboratory Test Interactions
- Changes in thyroxine binding globulin (TBG) concentration must be considered when interpreting levothyroxine and triiodothyronine values, which necessitates measurement and evaluation of unbound (free) hormone and/or determination of the free levothyroxine index. Pregnancy, infectious hepatitis, estrogens, estrogen containing oral contraceptives, and acute intermittent porphyria increase TBG concentrations. Decreases in TBG concentrations are observed in nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, and after androgen or corticosteroid therapy. Familial hyper or hypo thyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Pregnancy Category A – There are no reported cases of Levothyroxine Sodium for Injection used to treat myxedema coma in patients who were pregnant or lactating. Studies in pregnant women treated with oral levothyroxine to maintain a euthyroid state have not shown an increased risk of fetal abnormalities. Therefore, pregnant patients who develop myxedema should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the maternal patient and the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Levothyroxine (injection) in women who are pregnant.
Labor and Delivery
- Patients in labor who develop myxedema have not been reported in the literature. However, patients should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the maternal patient and the fetus.
Nursing Mothers
- Adequate replacement doses of thyroid hormones are required to maintain normal lactation. There are no reported cases of Levothyroxine Sodium for Injection used to treat myxedema coma in patients who are lactating. However, such patients should be treated with Levothyroxine Sodium for Injection as the risk of nontreatment is associated with a high probability of significant morbidity or mortality to the nursing patient.
Pediatric Use
- Myxedema coma is a disease of the elderly. An approved, oral dosage form of levothyroxine should be used in the pediatric patient population for maintaining a euthyroid state in non-complicated hypothyroidism.
Geriatic Use
- Because of the increased prevalence of cardiovascular disease in the elderly, cautious use of Levothyroxine Sodium for Injection in the elderly and in patients with known cardiac risk factors is advised. Atrial fibrillation is a common side effect associated with levothyroxine treatment in the elderly
Gender
There is no FDA guidance on the use of Levothyroxine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Levothyroxine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Levothyroxine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Levothyroxine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Levothyroxine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Levothyroxine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Levothyroxine (injection) in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Levothyroxine (injection) in the drug label.
Overdosage
- In general, the signs and symptoms of overdosage with levothyroxine are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Excessive doses of Levothyroxine Sodium for Injection (greater than 500 mcg) are associated with cardiac complications in patients with underlying cardiac disease.
Treatment of Overdosage
- Levothyroxine Sodium for Injection should be reduced in dose or temporarily discontinued if signs or symptoms of overdosage occur. To obtain up-to-date information about the treatment of overdose, a good resource is the certified Regional Poison Control Center. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in the patient.
- In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s medical status.
Pharmacology
There is limited information regarding Levothyroxine (injection) Pharmacology in the drug label.
Mechanism of Action
- Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and levothyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
- The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
Structure
- Levothyroxine Sodium for Injection contains synthetic crystalline levothyroxine (L-thyroxine) sodium salt. Levothyroxine sodium has an empirical formula of C15H10I4NNaO4, a molecular weight of 798.85 g/mol (anhydrous), and the following structural formula:
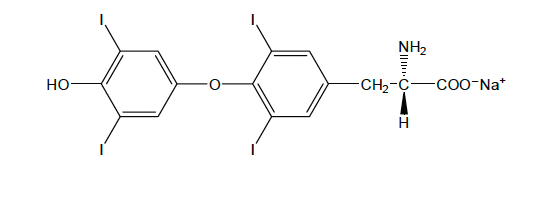
- Levothyroxine Sodium for Injection is a sterile, preservative-free lyophilized powder consisting of the active ingredient, levothyroxine sodium, and the excipients dibasic sodium phosphate heptahydrate, USP; mannitol, USP; and sodium hydroxide, NF in single-use amber glass vials. Levothyroxine Sodium for Injection is available at three dosage strengths: 100 mcg per vial, 200 mcg per vial and 500 mcg per vial.
Pharmacodynamics
- Thyroid hormone synthesis and secretion is regulated by the hypothalamic pituitary-thyroid axis. Thyrotropin releasing hormone (TRH) released from the hypothalamus stimulates secretion of thyrotropin stimulating hormone (TSH) from the anterior pituitary. TSH, in turn, is the physiologic stimulus for the synthesis and secretion of thyroid hormones, T4 and T3, by the thyroid gland. Circulating serum T3 and T4 levels exert a feedback effect on both TRH and TSH secretion. When serum T3 and T4 levels increase, TRH and TSH secretion decrease. When thyroid hormone levels decrease, TRH and TSH secretion increases. TSH is used for the diagnosis of hypothyroidism and evaluation of levothyroxine therapy adequacy with other laboratory and clinical data [see Dosage (2.1)]. There are drugs known to affect thyroid hormones and TSH by various mechanisms and those examples are diazepam, ethioamide, lovastatin, metoclopramide, 6-mercaptopurine, nitroprusside, perphenazine, and thiazide diuretics. Some drugs may cause a transient decrease in TSH secretion without hypothyroidism and those drugs (dose) are dopamine (greater than 1 mcg per kg per min), glucocorticoids (hydrocortisone greater than 100 mg per day or equivalent) and octreotide (greater than 100 mcg per day).
- Thyroid hormones regulate multiple metabolic processes and play an essential role in normal growth and development, and normal maturation of the central nervous system and bone. The metabolic actions of thyroid hormones include augmentation of cellular respiration and thermogenesis, as well as metabolism of proteins, carbohydrates and lipids. The protein anabolic effects of thyroid hormones are essential to normal growth and development.
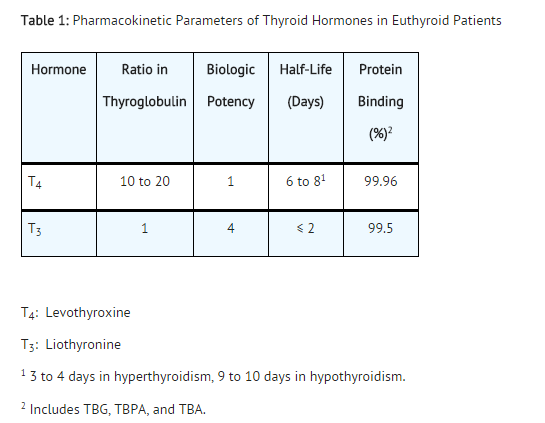
Pharmacokinetics
Absorption – Levothyroxine Sodium for Injection is administered via the intravenous route. Following administration, the synthetic levothyroxine cannot be distinguished from the natural hormone that is secreted endogenously.
Distribution – Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine binding globulin (TBG), thyroxine binding prealbumin (TBPA), and albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T4 partially explains the higher serum levels, slower metabolic clearance, and longer half life of T4 compared to T3. Protein bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Warnings and Precautions (5) and Drug Interactions (7)]. Thyroid hormones do not readily cross the placental barrier.
Metabolism – T4 is slowly eliminated. The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately eighty percent of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (r T3). T3 and r T3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Elimination – Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged, where it is hydrolyzed and eliminated in feces as the free hormones. Urinary excretion of T4 decreases with age.
Drug Interactions
- A listing of drug interaction with T4 is provided in the following tables, although it may not be comprehensive due to the introduction of new drugs that interact with the thyroidal axis or the discovery of previously unknown interactions. The prescriber should be aware of this fact and should consult appropriate reference sources (e.g., package inserts of newly approved drugs, medical literature) for additional information if a drug-drug interaction with levothyroxine is suspected.
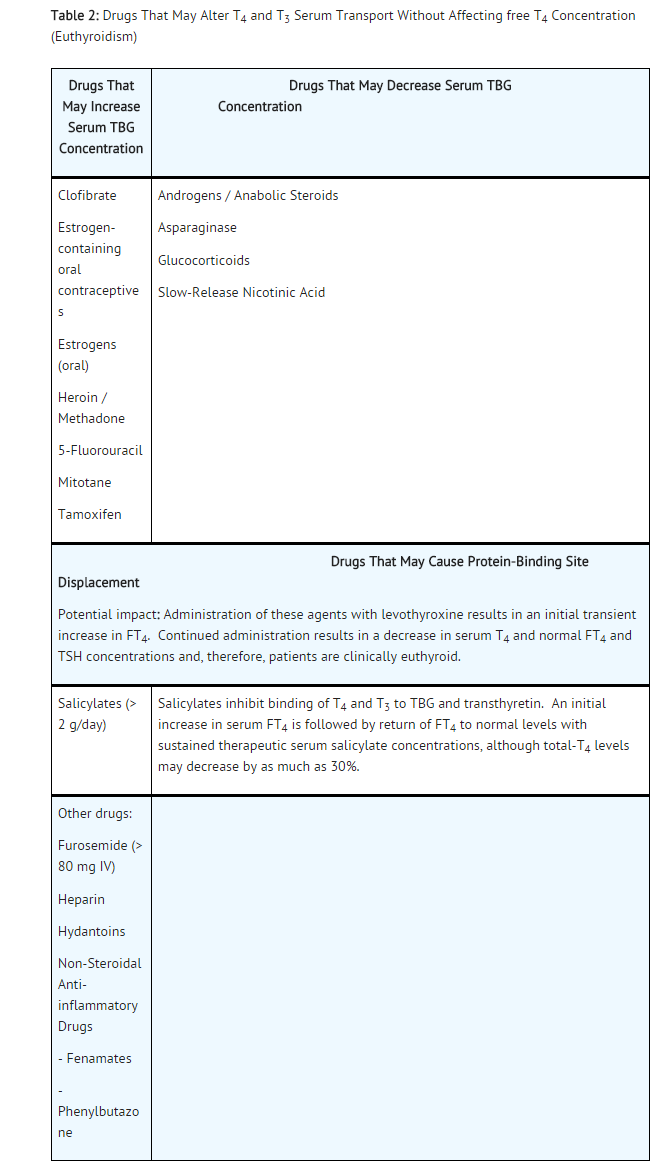
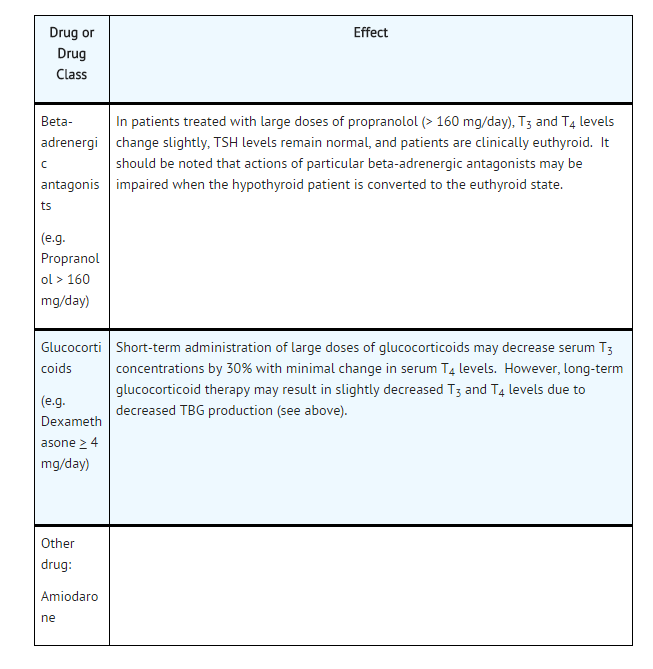
Table 3: Drugs That May Alter Hepatic Metabolism of T4 (Hypothyroidism)
Potential impact: Stimulation of hepatic microsomal drug-metabolizing enzyme activity may cause increased hepatic degradation of levothyroxine, resulting in increased levothyroxine requirements.

Table 4: Drugs That May Decrease Conversion of T4 to T3
Potential impact: Administration of these enzyme inhibitors decreases the peripheral conversion of T4 to T3, leading to decreased T3 levels. However, serum T4 levels are usually normal but may occasionally be slightly increased.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of Levothyroxine Sodium for Injection.
Animal Toxicology and Pharmacology
- No animal toxicology studies have been conducted with Levothyroxine Sodium for Injection.
Clinical Studies
- No clinical studies have been conducted with Levothyroxine Sodium for Injection in patients with myxedema coma. However, data from published literature support the intravenous use of levothyroxine sodium for the treatment of myxedema coma.
How Supplied
- Levothyroxine Sodium for Injection is available in three dosage strengths.
Storage
- Protect from light and store dry product at 20° to 25°C (68° to 77°F). Reconstituted drug product is preservative free. Discard any unused portion.
- This container closure is not made with natural rubber latex.
Images
Drug Images
{{#ask: Page Name::Levothyroxine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Levothyroxine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Levothyroxine (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Levothyroxine (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LEVOTHYROXINE SODIUM®[1]
Look-Alike Drug Names
There is limited information regarding Levothyroxine (injection) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.