Ventricular assist device
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Marv Slepian, M.D.; University of Arizona [2]; Juan A. Sanchez MD MPA [3], Chairman, The Stanley J. Dudrick Department of Surgery, Saint Mary's Hospital, Waterbury, CT, Tayebah Chaudhry[4], Syed Hassan A. Kazmi BSc, MD [5]
Overview
Ventricular assist device (VAD) is a mechanical device that provides Mechanical Circulatory Support (MCS) in patients with advanced heart failure. VAD therapy has shown improved outcome and survival in heart failure patients compared to pharmacological therapy alone. These devices are commonly used as a Bridge-to-Transplant (BTT) in heart transplant eligible patients or as a Destination Therapy (DT) in patients ineligible for heart transplant. VADs are indicated for either short-term use in patients recovering from heart attacks or heart surgery or for long-term use (months to years and in some cases for life) in patients suffering from end-stage heart failure.
Over the years, significant development has been made to create miniaturized VADs with increased durability, reliability and lesser noise emission. Newer (third generation) VADs are Continuous Flow (CF) pumps with lesser complications compared to the older (first generation) VADs that were large and had pulsatile-flow pumps. Depending on the heart chamber that needs MCS, VADs can be used as Left Ventricular Assist Devices (LVADs), Right Ventricular Assist Devices (RVADs) or BiVentricular Assist Devices (BiVADs).
Some VADs are percutaneous or transcutaneous (Impella, TandemHeart) while others are implantable (HeartMate II, HeartMate III), that allow patients to be fully mobile.
Ongoing research in VAD development is being focused on eliminating the need for a driveline, better surgical approaches and strategies for telemetric assessment of device function and remote charging of batteries.
History
VADs evolved over time after an external technology was used to provide cardiovascular support to a patient with Cardiopulmonary bypass (CPB) in an atrial septal defect repair surgery by John Gibbons. This lead to further interest and efforts to developing an artificial heart and later implantation of VAD in the late 1960s. Thoratec pneumatic VAD was the first Food and Drug Administration (FDA) approved VAD used as a BTT. This technology further evolved into HeartMate I, but more work needed to be done to develop newer LVADs with lesser complications. The Second and Third Generation VADs were designed to have a continuous flow rather than pulsatile. [1]
Types of Ventricular Assist Devices (VADs)
VADs are implanted in one ventricle or both ventricles of heart.
Left Ventricular Assist Device (LVAD)
- LVADs are the most commonly implanted VADs in end-stage heart failure patients. With robust innovation in technology, LVADs have significantly increased patient survival despite the adverse events associated with the devices. This has lead to growing proportion of patients undergoing LVAD implantations as a Destination therapy.
- LVADs unload left ventricle and augment the cardiac outflow (nearly 10L/min) into the ascending aorta.
- HeartMate II, HeartMate III and HeartWare HVAD are the only commercially FDA- approved LVADs for adults in North America. Worldwide efforts are being made to create the most effective and safest interface including the INCOR, Jarvik 2000, EVAHEART LVAS and more. [2]
Right Ventricular Assist Device (RVAD)
- RVADs may be needed in the management of Right ventricular failure (RVF) for mechanical circulatory support. RVF can be from a primary cause, for example, acute right ventricular infarction, increased afterload resulting from acute pulmonary embolism or chronic pulmonary hypertension, congenital heart disease, or it can be from a secondary cause, for example, post-cardiotomy (commonly as a complication of LVAD implantation). [3]
- RVADs are implanted surgically or percutaneously. Impella RP is an example of minimally invasive percutaneous RVAD. The pump has an inflow in the inferior vena cava and outflow in the pulmonary artery.
BiVentricular Assist Device (BiVAD)
- BiVAD is used to provide simultaneous MCS to both ventricles of heart. It is different from Total Artificial Heart (TAF) since it works in parallel to the native heart and removal of native heart is not required. Hence, BiVAD provides a potential for myocardial recovery of the retained native heart and reduces the need for a donor heart.[4]
- BiVAD may be indicated in right ventricular dysfunction after LVAD implantation. However, early and/or long-term dual VAD therapy for biventricular support may be considered in evident right ventricular failure and conditions like acute circulatory collapse due to fulminant myocarditis, acute decompensation of dilated biventricular cardiomyopathy, massive Myocardial Infarction (involving the septum or RV), acute deterioration following toxic cardiomyopathy, infiltrative cardiomyopathy or RV cardiomyopathies with concomitant involvement of the left ventricle.[5]
- Examples of VADs implanted as BiVAD include Abiomed BVS5000 and AB5000, Thoratec PVAD and IVAD, Berlin Heart, Levitronix CentriMag, Jarvik 2000 and HeartWare HVAD.
Classification
VADs are classified according to their operational characteristics. Most VADs operate on similar basic principle i.e., a cannula is inserted into the apex of the ventricle. Blood passes through this cannula to a pump and thence through a tube into the aorta (most commonly ascending aorta) in case of an LVAD or into the pulmonary artery in case of an RVAD. The pump is powered through a lead which connects it to a controller and power supply. In some cases there is also a tube to vent the pump to the outside air. Major distinguishing features between different VADs are the pumps (which vary substantially in method of operation, size and placement), the controller and the materials used for the pump and the associated parts (tubes, cannulas, lead between the pump and the controller/power supply).
First-generation devices
- First generation VADs were large volume-displacement pumps with pulsatile flow generated by electronic or pneumatic compression of blood chambers.
- Several factors lead to the discontinuation of first generation VADs. These VADs had poor durability due to membrane rupture, bearing, and mechanical valve wear in addition to the higher rate of common VAD complications[4]They had large control consoles that significantly reduced patient mobility and pump that was audible and caused discomfort. These VADs were also associated with higher risk of infection at external lead, significant hemolysis, thrombus formation, larger surgical incision requirement and severe postoperative bleeding. Examples include Paracorporeal Ventricular Assist Device, HeartMate XVE and Novacor.
- Paracorporeal Ventricular Assist Device had a pump that was powered pneumatically and placed outside the body. Blood was pulled out of left ventricle through cannulas by a vacuum, passed through a reservoir and returned to aorta creating a pulsatile flow.[6]
- HeartMate XVE had a pump that was powered electronically and placed in a pocket in the pre-peritoneal space. It was smaller in size than Paracorporeal VAD. Due to poor durability and bearing wear, device replacement was required in a significant percentage of patients.[6]
Second-generation devices
- Second Generation VADs are Continuous Flow (CF) devices that use rotary centrifugal, diagonal or axial impellers. Although there is marked improvement in design and patient mobility compared to the first generation VADs, their rotary pumps are associated with increased thrombogenicity and acquired platelet dysfunction due to the lack of pulsatile flow and high shear forces near the bearings and seals. Commonly used second generation VADs include HeartMate II and Jarvik 2000. These pumps are easier to implant due to their smaller design. These are also quieter and have lower rates of infection and other complications compared to First Generation VADs. [7]
- HeartMate II draws blood from left ventricular apex and pumps it into ascending aorta producing a non-pulsatile flow. It is about the size of a D battery with a rotating impeller which is the shape of an Archimedes screw. Impeller is the only moving part of the pump. Due to lesser adverse events HeartMate II was approved by FDA for BTT in 2008 and later for DT in 2010 due to improved pump durability and minimal damage to blood cells. [8]
- Jarvik 2000 Ventricular Assist System development started in 1987 with several improvements in design since then. The newer Jarvik 2000 pump is a miniaturized intraventricular pump that is placed in the cardiac apex with minimally invasive implantation procedures. It creates a blood flow towards the ascending or descending aorta through an outflow conduit and lacks a real inflow conduit. Older pumps required Cardiopulmonary Bypass (CPB) support but less invasive off-pump implantation is done now with or without ExtraCorporeal Membrane Oxygenation (ECMO) instead of CPB support. [9]
Third-generation devices
- Third Generation VADs are Continuous Flow (CF) centrifugal pumps that use contactless/bearingless impeller drive and suspension systems and hence, completely eliminate mechanical wear of the pump components. Impeller is levitated by electromagnetic and/or hydrodynamic forces while active electromagnetic forces are employed to rotate the impeller. These VADs are more durable, easier to implant and have lower risk of thrombosis and hemolysis. Examples include HeartMate III and HeartWare HVAD. [7]
- HeartMate III is the newer LVAD technology that has shown to have fewer complications like strokes, major bleeding, GI bleeding or ventricular arrhythmias when compared to HeartMate II. Fewer pump thromboses and LVAD replacements for LVAD malfunction have also been reported with this newest LVAD. The improved function of HeartMate III is due to it's higher hemocompatibility. It has wide blood flow conduits which reduce shear of the blood.[1] It's frictionless design without mechanical bearings not only reduces wear-and-tear but also decreases the amount of heat produced during impeller rotation. [10]
- HeartWare HVAD is placed intrapericardially in the left ventricular apex. It has a bearingless magnetically driven impeller. It is suitable for patients with smaller body surface area. Due to its small size, it has also been used as an implantable RVAD.[8]
Indications
VADs can be used for the following conditions: [1]
- Bridge-to-Transplant (BTT): Patients who are transplant eligible and need a donor heart for survival.
- Destination therapy (DT): Patients who need mechanical circulatory support as an alternative to heart transplant.
- Bridge-to-Decision (BTD): Patients who are not assigned the need for heart transplant at the time of implantation.
- Bridge-to-Candidacy (BTC): Patients who are not transplant eligible but may be reconsidered for transplant after a temporary circulatory support.
- Bridge-to-Recovery (BTR): Patients who require temporary circulatory support after an acute insult to cardiac function, like cardiogenic shock.
Modern indications for short-term use of MCS include: [10]
- Bridge-to-immediate survival
- Brdige-to-next decision
- Bridge-to-bridge
- Bridge-to-surgery
Contraindications
- Coexisting severe comorbidity like irreversible hepatic, renal, or neurological disease [1]
- Patient non-compliance [1]
- Severe psychosocial limitations (Unmanaged psychiatric disorders, lack of social support) [1]
- Aortic regurgitation (in case of LVAD) and Pulmonary regurgitation (in case of RVAD) [3]
- Limited life expectancy (age >80 for DT, untreated malignancy) [1]
- Poor nutritional status (obesity or malnutrition) [1]
- Limitations to rehabilitation (musculoskeletal disease, severe peripheral vascular disease, active substance abuse, active infection, prolonged intubation) [1]
Complications
- Right Ventricular(RV) failure:
Unloading of the left ventricle post-LVAD implantation may result in septal shifts, leading to increased RV preload, decreased RV contractility and function. RVAD implantation may be necessary until RV function improves, at which point RVAD can be explanted. [1]
- Bleeding:
Bleeding is a common complication during the implantation procedure. Patients with Continuous Flow VADs are systemically anticoagulated with Warfarin, which further increases the risk of bleeding, particularly Gastro-intestial (GI) Bleed. Patients should be evaluated and if possible, treated preoperatively for GI bleeding sources such as colonic polyps, stomach ulcers and angiodysplasias. There is also a higher risk of bleeding post-LVAD implantation from degradation of Von-Willebrand Factor (VWF) by VAD rotors, which is reversible once LVAD is removed.[1]
- Thromboemolic events and Stroke:
There is an increased risk of thromboembolic events and Transient Ischemic Attacks (TIAs)in patients with Continous Flow VADs despite of systemic anticoagulation. If pump thrombosis develops, there might be an emergent need for pump exchange and moving the patient up on priority list of heart transplant. [1]
- Infection:
Infection at multiple sites of VAD circuit is a common complication. These sites include the VAD pocket where the VAD is implanted, the VAD itself or the cannulas that go from the ventricle to the VAD and from the VAD to the aorta. The most common infection site is the driveline which goes from the VAD through the skin to the VAD power source since it is a pathway from the external environment to the VAD interior. Common causative organisms in driveline infections are the skin flora such as Staphylococcus aureus and coagulase-negative staphylococci and in infection of internal components are Serratia, Klebsiella, and Enteroccocus species, Pseudomonas aeruginosa. Candida can cause up to ten percent of infections. Treatment includes debridement of infected tissue and administration of intravenous antibiotics. Explantantion of infected LVAD is sometimes necessary. [1]
- Ventricular arrythmias:
Ventricular arrythmias is a common complication after LVAD implantation. It is thought to be from re-entrant circuits that are formed around the site of the inflow cannula in already damaged myocardium. Implantable cardioverter-defibrillator helps with these arrhythmias until the ventricles remodel as the time goes on. Anti-arrhythmic agents such as lidocaine or amiodarone are rarely necessary. [1]
- Device Malfunction and Optimization:
Malfunction of different parts of VAD ( driveline, controller, pump) can cause the device to stop working.[11] VADs are complex devices that need an expert to operate them in order to obtain optimal right and left ventricular hemodynamics.[1]
- Valvular Insufficiency:
Mitral and Aortic valve insufficiency can be seen post-LVAD implantation. This can effect pump function and may worsen heart failure. [11]
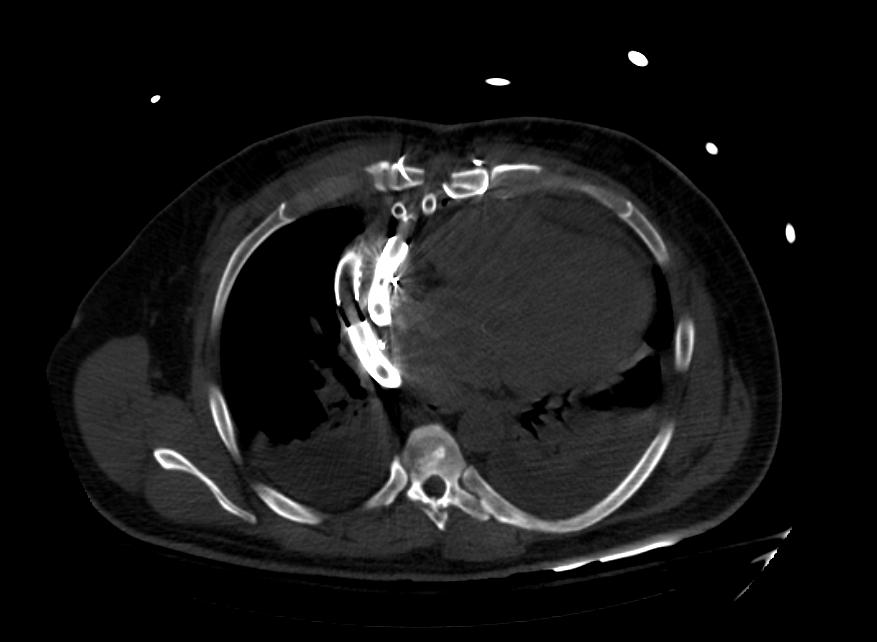
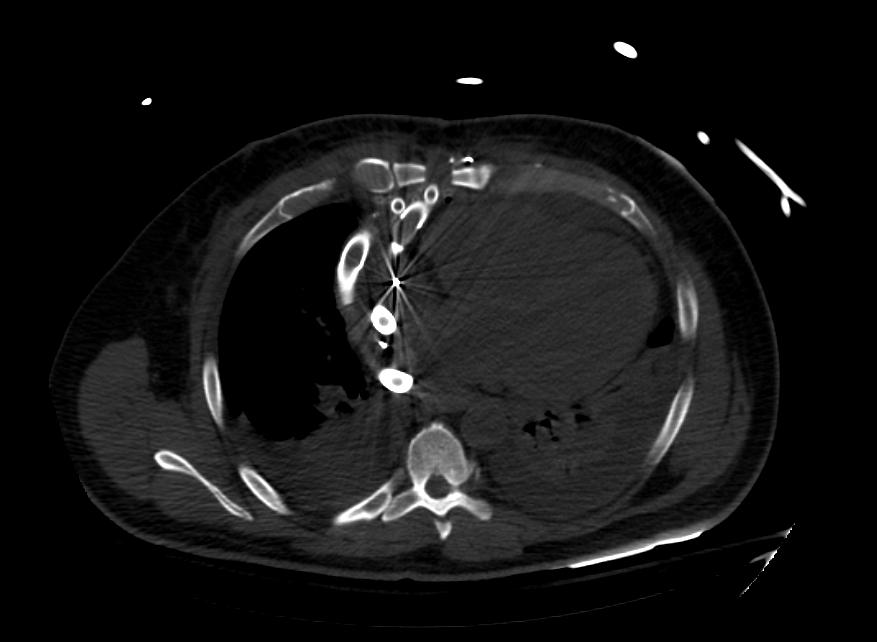
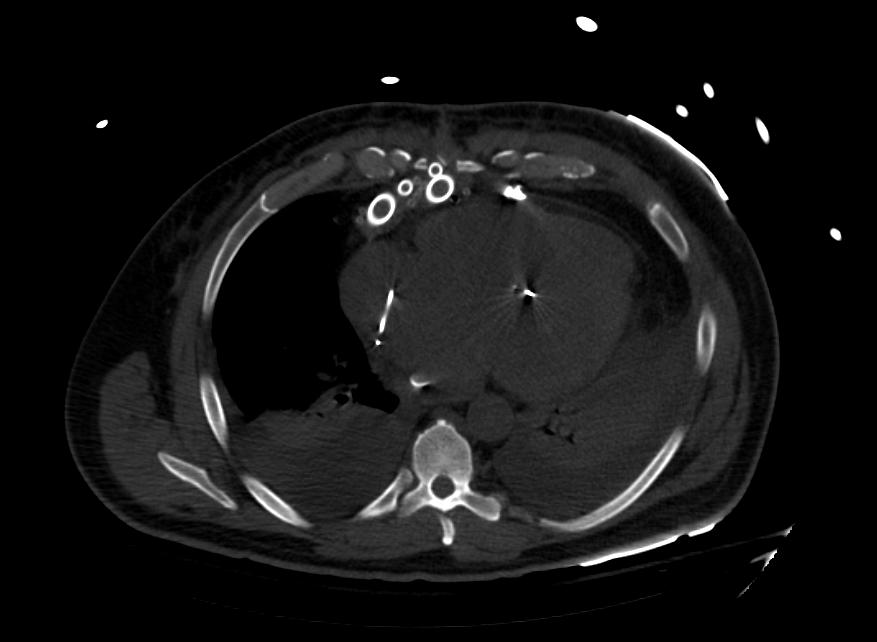
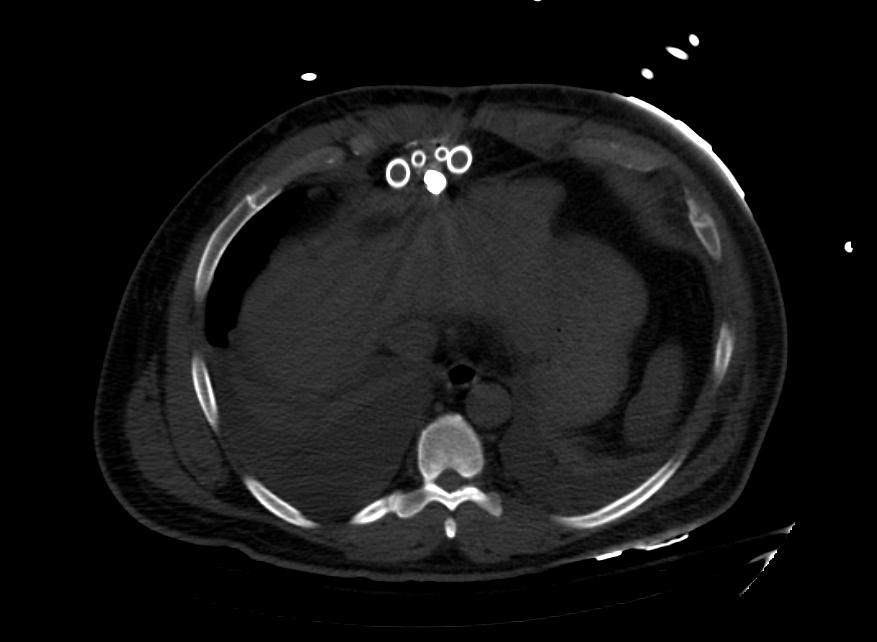
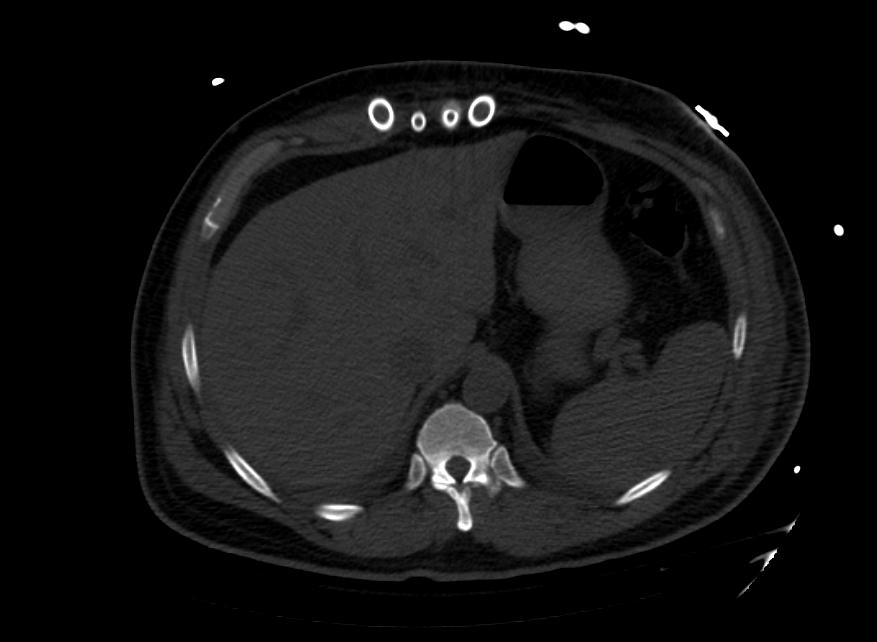
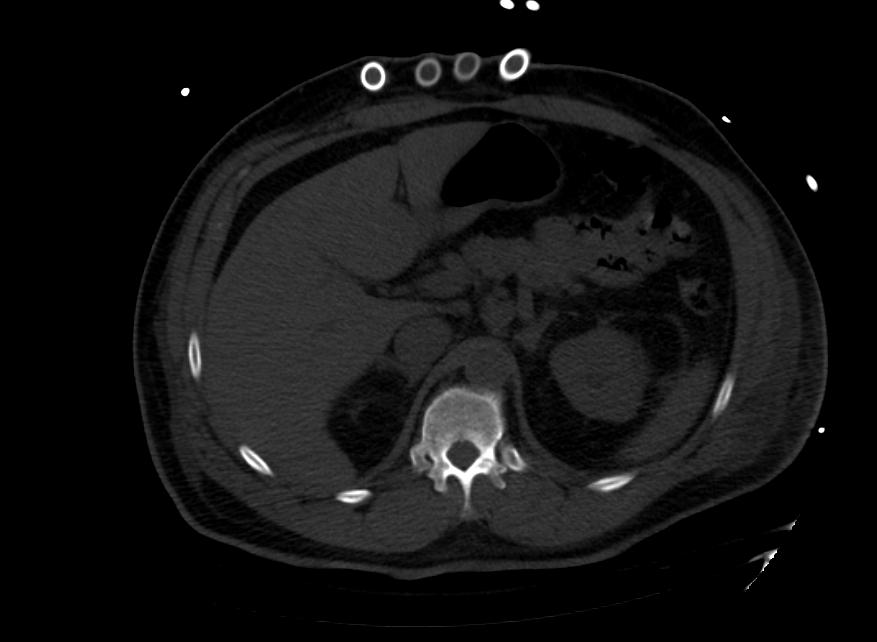
Images: Ventricular Assist Devices
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
Recent advances in LVAD Therapy with Percutaneous LVADs
Recent developments in LVAD therapy have been made to provide short-term temporary MCS to patients in cardiogenic shock. Axillary artery is commonly being used for insertion of temporary VADs to allow for improved patient mobility. [6]
- Intra-aortic balloon pump was the first temporary VAD that is still being used.
- ExtraCorporeal Membrane Oxygenation (ECMO) is being used to provide cardiac and pulmonary support.
- Tandem Heart is a left atrial to iliofemoral artery bypass pump.
- Impella is an axial flow pump with non-pulsatile blood flow from left ventricle to ascending aorta.
Fully Implantable Pumps
Most VADs currently being used are not fully implantable since they connect to an external power source, hence increasing the risk of infection. Fully implantable devices could reduce this risk of infection. The newest totally implantable LVAD (Leviticus FiVAD) uses the Jarvik VAD platform and is undergoing clinical trials. [1]
List of implantable VAD devices
This is a partial list and may never be complete
Referenced additions are welcome
| Device | Manufacturer | Type | Approval Status as at December 2007 |
|---|---|---|---|
| Novacor | World Heart | Pulsatile | Approved for use in North America, European Union and Japan |
| Heartmate | Thoratec | Pulsatile | Approved for use in North America |
| Heartmate II | Thoratec | Rotor driven continuous axial flow, ball and cup bearings. | Approved for use in European Union. FDA approval for bridge to transplant achieved November 2007. |
| Incor | Berlin Heart | Continuous flow driven by a magnetically suspended axial flow rotor. | Approved for use in European Union. |
| Jarvik 2000 | Jarvik Heart | Continuous flow, axial rotor supported by ceramic bearings | Approved for use in the European Union. Clinical trials for FDA approval are planned. |
| MicroMed DeBakey VAD | MicroMed | Continuous flow driven by axial rotor supported by ceramic bearings | Approved for use in the European Union. The child version is approved for use in children in USA. Undergoing clinical trials in USA for FDA approval. |
| VentrAssist | Ventracor | Continuous flow driven by a hydrodynamically suspended centrifugal rotor. | Approved for use in European Union and Australia. Undergoing clinical trials for FDA approval |
| MTIHeartLVAD | MiTiHeart Corporation | Continuous flow driven by a magnetically suspended centrifugal rotor. | Yet to start clinical trials. |
| C-Pulse | Sunshine Heart | Pulsatile, driven by an inflatable cuff around the aorta | Yet to start clinical trials |
| HVAD | HeartWare | Similar to Ventracor - magnetic impellor similar to BLDCmotor | undergoing clinical trials for CE, applied to start FDA US trials
|
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Eisen HJ (July 2019). "Left Ventricular Assist Devices (LVADS): History, Clinical Application and Complications". Korean Circ J. 49 (7): 568–585. doi:10.4070/kcj.2019.0161. PMC 6597447 Check
|pmc=value (help). PMID 31243930. - ↑ Han JJ, Acker MA, Atluri P (December 2018). "Left Ventricular Assist Devices". Circulation. 138 (24): 2841–2851. doi:10.1161/CIRCULATIONAHA.118.035566. PMID 30565993.
- ↑ 3.0 3.1 Pieri M, Pappalardo F (October 2018). "Impella RP in the Treatment of Right Ventricular Failure: What We Know and Where We Go". J. Cardiothorac. Vasc. Anesth. 32 (5): 2339–2343. doi:10.1053/j.jvca.2018.06.007. PMID 30093192.
- ↑ 4.0 4.1 Gregory, Shaun D.; Timms, Daniel; Gaddum, Nicholas; Mason, David G.; Fraser, John F. (2011). "Biventricular Assist Devices: A Technical Review". Annals of Biomedical Engineering. 39 (9): 2313–2328. doi:10.1007/s10439-011-0348-8. ISSN 0090-6964.
- ↑ Hayward, Christopher S; Shehab, Sajad (2019). "Choosing Between Left Ventricular Assist Devices and Biventricular Assist Devices". Cardiac Failure Review. 5 (1): 19. doi:10.15420/cfr.2018.23.2. ISSN 2057-7540.
- ↑ 6.0 6.1 6.2 Miller LW, Rogers JG (July 2018). "Evolution of Left Ventricular Assist Device Therapy for Advanced Heart Failure: A Review". JAMA Cardiol. 3 (7): 650–658. doi:10.1001/jamacardio.2018.0522. PMID 29710092.
- ↑ 7.0 7.1 Vaidya Y, Patibandla S, Dhamoon AS. PMID 29763016. Missing or empty
|title=(help) - ↑ 8.0 8.1 Stone ME, Pawale A, Ramakrishna H, Weiner MM (August 2018). "Implantable Left Ventricular Assist Device Therapy-Recent Advances and Outcomes". J. Cardiothorac. Vasc. Anesth. 32 (4): 2019–2028. doi:10.1053/j.jvca.2017.11.003. PMID 29338999.
- ↑ Zucchetta F, Tarzia V, Bottio T, Gerosa G (September 2014). "The Jarvik-2000 ventricular assist device implantation: how we do it". Ann Cardiothorac Surg. 3 (5): 525–31. doi:10.3978/j.issn.2225-319X.2014.09.09. PMC 4229469. PMID 25452914.
- ↑ 10.0 10.1 Stone, Marc E.; Pawale, Amit; Ramakrishna, Harish; Weiner, Menachem M. (2018). "Implantable Left Ventricular Assist Device Therapy—Recent Advances and Outcomes". Journal of Cardiothoracic and Vascular Anesthesia. 32 (4): 2019–2028. doi:10.1053/j.jvca.2017.11.003. ISSN 1053-0770.
- ↑ 11.0 11.1 Emani S (July 2018). "Complications of Durable Left Ventricular Assist Device Therapy". Crit Care Clin. 34 (3): 465–477. doi:10.1016/j.ccc.2018.03.003. PMID 29907277.
External links
- Nader Moazami, Patrick M. McCarthy Temporary Circulatory Support
- Eugene L. Kukuy, Mehmet C. Oz, Yoshifumi Naka Long-Term Mechanical Circulatory Support - a review of the subject as at 2003.
- Health Center Online VAD
- Mayo Clinic VAD
- FDA VAD
- Life without a pulse — news story about Canadian man with VAD
- Heart Pump Design Could Give Patients New Hope — A new counter-flow heart pump developed by Queensland University of Technology
- Heart pump improves quality of life in congestive heart failure patientsA rapid review of the medical literature and specialist opinion as at December 2005
- NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE (UK) Interventional procedures overview - short-term circulatory support with left ventricular assist devices as a bridge to cardiac transplantation or recovery A rapid review of the medical literature and specialist opinion as at December 2005
- Courtney J. Gemmato,Matthew D. Forrester,Timothy J. Myers,O.H. Frazier,Denton A. Cooley Thirty-Five Years of Mechanical Circulatory Support at the Texas Heart InstituteTex Heart Inst J 2005;32:168-77