Kanamycin microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Microbiology
Kanamycin is a bactericidal antibiotic which acts by inhibiting the synthesis of protein in susceptible microorganisms. Kanamycin sulfate is active in vitro against many strains of Staphylococcus aureus (including penicillinase and non penicillinase-producing strains), Staphylococcus epidermidis, N. gonorrhoeae, H. influenzae, E. coli, Enterobactor aerogenes, Shigella and Salmonella species, K. pneumoniae, Serratia marcescens, Providencia species, Acinetobacter species and Citrobacter freundii and Citrobacter species, and many strains of both indole-positive and indole-negative Proteus strains that are frequently resistant to other antibiotics.
Aminoglycosides have a low order of activity against most gram-positive organisms including Streptococcus pyogenes, Streptococcus pneumoniae and enterococci. In vitro studies have demonstrated that an aminoglycoside combined with an antibiotic which interferes with cell wall synthesis (i.e., Penicillin G or ampicillin) affects some Group D streptococcal strains synergistically. Bacteriological testing and tests for antibiotic synergism are necessary.
Enzymatic inactivation of deoxystreptamine is the principal mechanism of resistance.
Susceptibility Testing
Quantitative methods for susceptibility testing that require measurement of zone diameters give the most precise estimates of antibiotic susceptibility. One such procedure has been recommended for use with discs to test susceptibility to kanamycin. Interpretation involves correlation of the diameters obtained in the disc test with minimal inhibitory concentration (MIC) values for kanamycin.
Reports from the laboratory give results of the standardized single disc susceptibility test (Bauer, et al., Am J Clin Path 1966;45:493 and Federal Register 37:20525-20529, 1972), using a 30 mcg kanamycin disc should be interpreted according to the following criteria:
Organisms producing zones of 18 mm or greater, or MIC’s of 16 mcg or less are considered susceptible, indicating that the test organism is likely to respond to therapy. Resistant organisms produce zones of 14 mm or less or MIC’s of 16 mcg or greater. A report of “resistant” from the laboratory indicates that the infecting organism is not likely to respond to therapy.
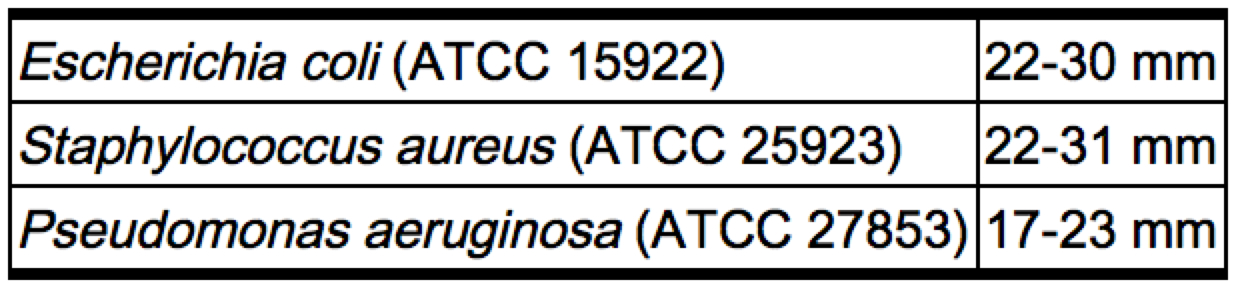
Zones greater than 14 mm and less than 18 mm, or MIC’s of greater than 16 mcg and less than 65 mcg, indicate intermediate susceptibility. A report of “intermediate” susceptibility suggests that the organism would be susceptible if the infection is confined to tissues and fluids (e.g., urine), in which high antibiotic levels are attained. Control organisms are recommended for susceptibility testing. Each time the test is performed one or more of the following organisms should be included: Escherichia coli ATCC 15922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853. The control organisms should produce zones of inhibition within the following ranges:[1]
 |
References
Adapted from the FDA Package Insert.