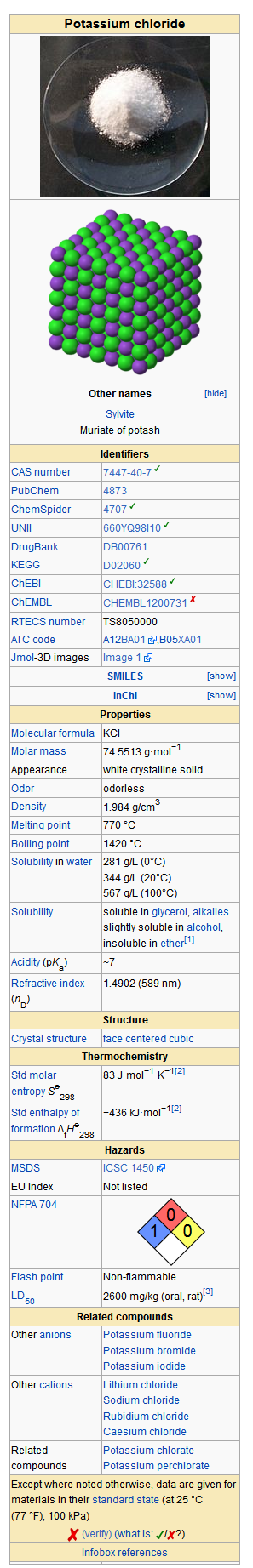
Potassium chloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Potassium chloride is a {{{drugClass}}} that is FDA approved for the treatment of hypokalemia with or without metabolic alkalosis; in digitalis intoxication ; in patients with hypokalemic familial periodic paralysis and for prevention of potassium depletion. Common adverse reactions include diarrhea, flatulance, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypokalemia with or without metabolic alkalosis
- For therapeutic use in patients with hypokalemia with or without metabolic alkalosis; in digitalis intoxication and in patients with hypokalemic familial periodic paralysis.
- Dosing Information
- Dosage must be adjusted to the individual needs of each patient but is typically in the range of 40-100 mEq per day or more for the treatment of potassium depletion.
- The usual adult dose is 20-100 mEq of potassium per day (one KLOR-CON® 20 mEq packet 1 to 5 times daily after meals or one KLOR-CON® /25 25 mEq packet 1 to 4 times daily after meals).
- The contents of each KLOR-CON® packet should be dissolved in at least 4 ounces of cold water or other beverage. The contents of each KLOR-CON® /25 packet should be dissolved in at least 5 ounces of cold water or other beverage. These preparations, like other potassium supplements, must be properly diluted to avoid the possibility of gastrointestinal irritation.
Hypokalemia; Prophylaxis
- Dosing Information
- Dosage must be adjusted to the individual needs of each patient but is typically in the range of 20 mEq per day for the prevention of hypokalemia.
- The use of potassium salts in patients receiving diuretics for uncomplicated essential hypertension is often unnecessary when such patients have a normal dietary pattern. Serum potassium should be checked periodically, however, and if hypokalemia occurs, dietary supplementation with potassium-containing foods may be adequate to control milder cases. In more severe cases supplementation with potassium salts may be indicated.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Potassium chloride in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Potassium chloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Potassium chloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Hypokalemia
- Developed by:
- Class of Recommendation: Pediatric, Class IIa
- Strength of Evidence: Pediatric, Category C
- Dosing Information
- The recommended dose of potassium chloride in children is 3 to 8 mEq/kg/day orally divided 1 to 5 times per day depending on tolerability and dose. Start at a lower dose and adjust based on serum potassium concentrations.
- The recommended dose of potassium chloride in neonates is 1 to 2 mEq/kg/24 hours orally. Monitor potassium concentrations.
Supplementation with Diuretics:
- When potassium chloride is administered concomitantly with diuretics, the recommended oral dose of potassium chloride is 1 to 3 mEq/kg/day
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Potassium chloride in pediatric patients.
Contraindications
- Potassium supplements are contraindicated in patients with hyperkalemia since a further increase in serum potassium concentration in such patients can produce cardiac arrest. Hyperkalemia may complicate any of the following conditions: chronic renal failure, systemic acidosis such as diabetic acidosis, acute dehydration, extensive tissue breakdown as in severe burns, adrenal insufficiency, or the administration of a potassium-sparing diuretic (e.g.,spironolactone, triamterene or amiloride). Contraindicated in persons demonstrating allergy to any of the components of the powder.
Warnings
Hyperkalemia
- In patients with impaired mechanisms for excreting potassium, the administration of potassium salts can produce hyperkalemia and cardiac arrest. This occurs most commonly in patients given potassium by the intravenous route but may also occur in patients given potassium orally. Potentially fatal hyperkalemia can develop rapidly and be asymptomatic.
- The use of potassium salts in patients with chronic renal disease, or any other condition which impairs potassium excretion, requires particularly careful monitoring of the serum potassium concentration and appropriate dosage adjustments.
Interaction with Potassium-Sparing Diuretics
- Hypokalemia should not be treated by the concomitant administration of potassium salts and a potassium-sparing diuretic (e.g., spironolactone, triamterene or amiloride), since the simultaneous administration of these agents can produce severe hyperkalemia.
Metabolic Acidosis
- Hypokalemia in patients with metabolic acidosis should be treated with an alkalinizing potassium salt such as potassium bicarbonate, potassium citrate or potassium acetate.
Precautions
General
- The diagnosis of potassium depletion is ordinarily made by demonstrating hypokalemia in a patient with a clinical history suggesting some cause for potassium depletion.
Laboratory Tests
- In interpreting the serum potassium level, the physician should be aware that acute alkalosis per se can produce hypokalemia in the absence of a deficit in total body potassium while acute acidosis per se can increase the serum potassium concentration into the normal range even in the presence of a reduced total body potassium. The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease, or acidosis, requires careful attention to acid-base balance and appropriate monitoring of serum electrolytes, the electrocardiogram, and the clinical status of the patient.
Adverse Reactions
Clinical Trials Experience
- There is limited information regarding Clinical Trial Experience of Potassium chloride in the drug label.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Potassium chloride in the drug label
Drug Interactions
There is limited information regarding Potassium chloride Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with potassium chloride. It is not known if potassium chloride causes fetal harm when administered to a pregnant woman or affects reproductive capacity. Potassium chloride should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Potassium chloride in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Potassium chloride during labor and delivery.
Nursing Mothers
- Many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from oral potassium supplements, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
- There is no FDA guidance on the use of Potassium chloride with respect to geriatric patients.
Gender
- There is no FDA guidance on the use of Potassium chloride with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Potassium chloride with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Potassium chloride in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Potassium chloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Potassium chloride in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Potassium chloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Dosage must be adjusted to the individual needs of each patient but is typically in the range of 20 mEq per day for the prevention of hypokalemia to 40-100 mEq per day or more for the treatment of potassium depletion.
- The usual adult dose is 20-100 mEq of potassium per day (one KLOR-CON® 20 mEq packet 1 to 5 times daily after meals or one KLOR-CON® /25 25 mEq packet 1 to 4 times daily after meals).
- The contents of each KLOR-CON® packet should be dissolved in at least 4 ounces of cold water or other beverage. The contents of each KLOR-CON® /25 packet should be dissolved in at least 5 ounces of cold water or other beverage. These preparations, like other potassium supplements, must be properly diluted to avoid the possibility of gastrointestinal irritation.
Monitoring
- There is limited information regarding Monitoring of Potassium chloride in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Potassium chloride in the drug label.
Overdosage
There is limited information regarding Potassium chloride overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Potassium ion is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes, including the maintenance of intracellular tonicity, the transmission of nerve impulses, the contraction of cardiac, skeletal, and smooth muscle and the maintenance of normal renal function.
- Potassium depletion may occur whenever the rate of potassium loss through renal excretion and/or loss from the gastrointestinal tract exceeds the rate of potassium intake. Such depletion usually develops slowly as a consequence of prolonged therapy with oral diuretics, primary or secondary hyperaldosteronism, diabetic ketoacidosis, severe diarrhea, or inadequate replacement of potassium in patients on prolonged parenteral nutrition. Potassium depletion due to these causes is usually accompanied by a concomitant deficiency of chloride and is manifested by hypokalemia and metabolic alkalosis. Potassium depletion may produce weakness, fatigue, disturbances of cardiac rhythm (primarily ectopic beats), prominent U-waves in the electro-cardiogram, and in advanced cases flaccid paralysis and/or impaired ability to concentrate urine.
- Potassium depletion associated with metabolic alkalosis is managed by correcting the fundamental causes of the deficiency whenever possible and administering supplemental potassium chloride, in the form of high potassium food or potassium chloride solution or tablets.
- In rare circumstances (e.g., patients with renal tubular acidosis) potassium depletion may be associated with metabolic acidosis and hyperchloremia. In such patients potassium replacement should be accomplished with potassium salts other than the chloride, such as potassium bicarbonate, potassium citrate or potassium acetate.
Structure
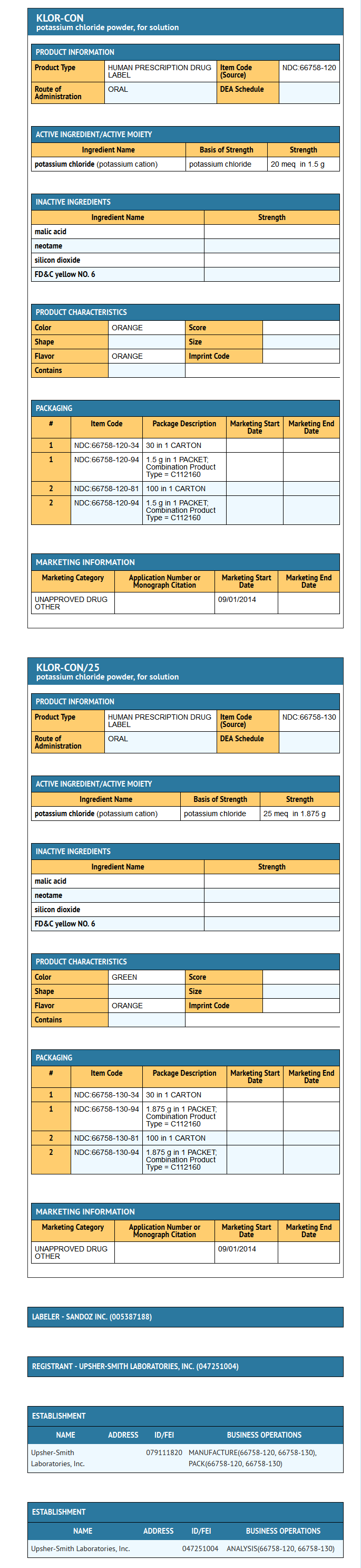
- Fruit-flavored KLOR-CON® and KLOR-CON®/25 Powder (Potassium Chloride for Oral Solution, USP) are oral potassium supplements offered as powder for reconstitution in individual packets. Each packet of KLOR-CON® powder contains potassium 20 mEq and chloride 20 mEq provided by potassium chloride 1.5 g. Each packet of KLOR-CON®/25 powder contains potassium 25 mEq and chloride 25 mEq provided by potassium chloride 1.875 g. KLOR-CON® and KLOR-CON®/25 are sugar-free. Inactive ingredients: FD&C Yellow No. 6, malic acid, neotame, silicon dioxide, and natural and/or artificial flavors.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Potassium chloride in the drug label.
Pharmacokinetics
- There is limited information regarding Pharmacokinetics of Potassium chloride in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity, mutagenicity and fertility studies in animals have not been performed. Potassium is a normal dietary constituent.
Clinical Studies
- There is limited information regarding Clinical Studies of Potassium chloride in the drug label.
How Supplied
- KLOR-CON® Powder (Potassium Chloride for Oral Solution, USP) 20 mEq is supplied in cartons of 30 packets (NDC 66758-120-34) and cartons of 100 packets (NDC 66758-120-81). Each packet contains potassium 20 mEq and chloride 20 mEq provided by potassium chloride 1.5 g.
- KLOR-CON® /25 Powder (Potassium Chloride for Oral Solution, USP) 25 mEq is supplied in cartons of 30 packets (NDC 66758-130-34) and cartons of 100 (NDC 66758-130-81). Each packet contains potassium 25 mEq and chloride 25 mEq provided by potassium chloride 1.875g.
Storage
- Store at controlled room temperature, 15-30°C (59-86°F).
Images
Drug Images

{{#ask: Page Name::Potassium chloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Potassium chloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Patient Counseling Information of Potassium chloride in the drug label.
Precautions with Alcohol
- Alcohol-Potassium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
K-Tab K-Dur Micro-K, Klor-Con, K-Lor, Kaon-CL, Kay Ciel, Klotrix
Look-Alike Drug Names
- A® — B®[1]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Potassium chloride
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Potassium chloride |Label Name=Potassium chloride11.png
}}
{{#subobject:
|Label Page=Potassium chloride |Label Name=Potassium chloride11.png
}}