Iobenguane I 123
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Iobenguane I 123 is a diagnostic agent that is FDA approved for the diagnosis of neuroblastoma,phaeochromocytoma,heart failure. Common adverse reactions include flushing , Injection site hemorrhage, Injection site reaction, pruritus , rash, dizziness and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Pheochromocytoma and Neuroblastoma
- AdreView is a radiopharmaceutical indicated for use in the detection of primary or metastatic pheochromocytoma or neuroblastoma as an adjunct to other diagnostic tests.
Congestive Heart Failure
AdreView is indicated for scintigraphic assessment of sympathetic innervation of the myocardium by measurement of the heart to mediastinum (H/M) ratio of radioactivity uptake in patients with New York Heart Association (NYHA) class II or class III heart failure and left ventricular ejection fraction (LVEF) ≤ 35%. Among these patients, AdreView may be used to help identify patients with lower one and two year mortality risks, as indicated by an H/M ratio ≥ 1.6.
Limitations of Use:
- In patients with congestive heart failure, AdreView utility has not been established for:
- Selecting a therapeutic intervention or for monitoring the response to therapy;
using the H/M ratio to identify a patient with a high risk for death.
Dosage
- For adults (≥ 16 years of age), the recommended dose is 10 mCi (370 MBq)
Radiation Dosimetry
The estimated absorbed radiation doses to adults from intravenous administration of AdreView are as shown in Table 2:

DOSAGE FORMS AND STRENGTHS
- Single use vials containing 5 mL solution for intravenous injection (2 mCi/mL at calibration time).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Iobenguane I 123 in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Iobenguane I 123 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Pheochromocytoma and Neuroblastoma
- AdreView is a radiopharmaceutical indicated for use in the detection of primary or metastatic pheochromocytoma or neuroblastoma as an adjunct to other diagnostic tests.
Congestive Heart Failure
AdreView is indicated for scintigraphic assessment of sympathetic innervation of the myocardium by measurement of the heart to mediastinum (H/M) ratio of radioactivity uptake in patients with New York Heart Association (NYHA) class II or class III heart failure and left ventricular ejection fraction (LVEF) ≤ 35%. Among these patients, AdreView may be used to help identify patients with lower one and two year mortality risks, as indicated by an H/M ratio ≥ 1.6.
Limitations of Use:
- In patients with congestive heart failure, AdreView utility has not been established for:
- Selecting a therapeutic intervention or for monitoring the response to therapy;
using the H/M ratio to identify a patient with a high risk for death.
Dosage
- For pediatric patients < 16 years of age weighing ≥ 70 kg, the recommended dose is 10 mCi (370 MBq).
- For pediatric patients < 16 years of age weighing < 70 kg, the recommended dose should be calculated according to patient body weight as shown in Table 1. The benzyl alcohol in AdreView may cause serious adverse reactions in premature or low birth-weight infants.

Radiation Dosimetry
The estimated absorbed radiation doses for children from intravenous administration of AdreView are as shown in Table 2:

DOSAGE FORMS AND STRENGTHS
- Single use vials containing 5 mL solution for intravenous injection (2 mCi/mL at calibration time).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Iobenguane I 123 in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Iobenguane I 123 in pediatric patients.
Contraindications
- AdreView is contraindicated in patients with known hypersensitivity to iobenguane or iobenguane sulfate.
Warnings
Hypersensitivity Reactions
- Hypersensitivity reactions have been reported following AdreView administration. Prior to administration, question the patient for a history of prior reactions to iodine, an iodine-containing contrast agent or other products containing iodine. If the patient is known or strongly suspected to have hypersensitivity to iodine, an iodine-containing contrast agent or other products containing iodine, the decision to administer AdreView should be based upon an assessment of the expected benefits compared to the potential hypersensitivity risks. Have anaphylactic and hypersensitivity treatment measures available prior to AdreView administration.
Imaging Errors due to Concomitant Medications and Risks Associated with Withdrawal of Medications
- Many medications have the potential to interfere with AdreView imaging and review of the patient's medications is required prior to AdreView dosing due to the risk for unreliable imaging results. If the AdreView imaging information is essential for clinical care, consider the withdrawal of the following categories of medications if the withdrawal can be accomplished safely: antihypertensives that deplete norepinephrine stores or inhibit reuptake (e.g., reserpine, labetalol), antidepressants that inhibit norepinephrine transporter function (e.g., amitriptyline and derivatives, imipramine and derivatives, selective serotonin reuptake inhibitors), and sympathomimetic amines (e.g., phenylephrine, phenylpropanolamine, pseudoephedrine and ephedrine). The period of time necessary to discontinue any specific medication prior to AdreView dosing has not been established.
Pheochromocytoma and Neuroblastoma
- Drugs which interfere with norepinephrine uptake in neuroendocrine tumors may lead to false negative imaging results. When medically feasible, stop these drugs before AdreView administration and monitor patients for the occurrence of clinically significant withdrawal symptoms, especially patients with elevated levels of circulating catecholamines and their metabolites.
Congestive Heart Failure
- Many commonly used cardiovascular, pulmonary, and neuropsychiatric medications interfere with AdreView imaging (see above). AdreView imaging should not be performed if discontinuation of these medications would involve risks which outweigh the value of AdreView imaging. In clinical trials, patients were not eligible for AdreView imaging if they were receiving medications in the above categories and the risks for medication withdrawal were unacceptable or if they were not clinically stable (e.g., experiencing continuing chest pain, hemodynamic instability, or clinically significant arrhythmia).
Risks for Benzyl Alcohol Toxicity in Infants
- AdreView contains benzyl alcohol at a concentration of 10.3 mg/mL. Benzyl alcohol has been associated with a fatal "Gasping Syndrome" in premature infants and infants of low birth weight. Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol.
- Observe infants for signs or symptoms of benzyl alcohol toxicity following AdreView administration. AdreView safety and effectiveness have not been established in neonates (pediatric patients below the age of 1 month).
Increased Radiation Exposure in Patients with Severe Renal Impairment
- AdreView is cleared by glomerular filtration and is not dialyzable. The radiation dose to patients with severe renal impairment may be increased due to the delayed elimination of the drug. Delayed AdreView clearance may also reduce the target to background ratios and decrease the quality of scintigraphic images. These risks importantly may limit the role of AdreView in the diagnostic evaluation of patients with severe renal impairment. AdreView safety and efficacy have not been established in these patients.
Imaging Errors due to Conditions that Affect the Sympathetic Nervous System
- Individuals with conditions that affect the sympathetic nervous system, e.g., Parkinsonian syndromes such as Parkinson's disease or multiple system atrophy, may show decreased cardiac uptake of AdreView independent of heart disease.
Thyroid Accumulation
- Failure to block thyroid uptake of iodine 123 may result in an increased long term risk for thyroid neoplasia.
Hypertension
- Assess the patient's pulse and blood pressure before and intermittently for 30 minutes after AdreView administration. AdreView may increase release of norepinephrine from chromaffin granules and produce a transient episode of hypertension, although this was not observed in the clinical studies. Prior to AdreView administration, ensure emergency cardiac and anti-hypertensive treatments are readily available.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- During clinical development 1346 patients were exposed to AdreView, 251 patients with known or suspected pheochromocytoma or neuroblastoma, 985 patients with heart failure, and 110 control patients. All patients were monitored for adverse reactions over a 24 hour period following AdreView administration.
Pheochromocytoma and Neuroblastoma
- Serious adverse reactions were not observed in the AdreView clinical study. Adverse reactions were all mild to moderate in severity and were predominantly isolated occurrences (≤ 2 patients) of one of the following reactions: dizziness, rash, pruritus, flushing or injection site hemorrhage.
Congestive Heart Failure
- No serious adverse reactions to AdreView were observed in clinical studies. Adverse reactions that occurred with a frequency > 1% were associated with the injection site (1.3%), problems such as hematoma and bruising. The other most common reactions were flushing (0.3%) and headache (0.4%). The adverse reactions were predominantly of mild to moderate intensity.
Postmarketing Experience
- Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions have uncommonly been reported during the postmarketing use of AdreView.
Drug Interactions
- The following drugs have the potential to decrease the uptake of norepinephrine and cause false negative imaging results: antihypertensives that deplete norepinephrine stores or inhibit reuptake (e.g., reserpine, labetalol), antidepressants that inhibit norepinephrine transporter function (e.g., amitriptyline and derivatives, imipramine and derivatives, selective serotonin reuptake inhibitors), sympathomimetic amines (e.g., phenylephrine, phenylpropanolamine, pseudoephedrine and ephedrine), and cocaine. Clinical studies have not determined which specific drugs may cause false negative imaging results nor whether all drugs in any specific pharmacologic class have the same potential to produce the negative imaging results. Increasing the dose of AdreView will not overcome any potential uptake limiting effect of these drugs. Before AdreView administration, discontinue (for at least 5 biological half-lives) drugs known or expected to reduce norepinephrine uptake, as clinically tolerated.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Pregnancy Category C: Any radiopharmaceutical, including AdreView, has a potential to cause fetal harm. It is not known whether AdreView can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Animal reproduction studies have not been conducted with AdreView. AdreView should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Iobenguane I 123 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Iobenguane I 123 during labor and delivery.
Nursing Mothers
- It is not known whether AdreView is excreted into human milk. However, iodine 123 is excreted into human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to interrupt nursing after administration of AdreView or not to administer AdreView, taking into account the importance of the drug to the mother. Based on the physical half-life of iodine 123 (13.2 hours) nursing women may consider interrupting nursing for 6 days after AdreView administration in order to minimize risks to nursing infants.
Pediatric Use
- The safety and effectiveness of AdreView have been established in the age groups 1 month to 16 years in patients with known or suspected neuroblastoma [see CLINICAL STUDIES (14.1)]. Safety and effectiveness in pediatric patients below the age of 1 month or in any pediatric patient with congestive heart failure have not been established
Geriatic Use
- In clinical studies of AdreView in heart disease, 27% of subjects were 65-74 years of age and 17% of subjects were 75 years of age or over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- AdreView is excreted by the kidneys, and the risks of adverse reactions, increased radiation dose, and occurrence of falsely negative imaging results, may be greater in patients with severely impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and image interpretation. Consider assessment of renal function in elderly patients prior to AdreView administration.
Gender
There is no FDA guidance on the use of Iobenguane I 123 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Iobenguane I 123 with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Iobenguane I 123 in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Iobenguane I 123 in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Iobenguane I 123 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Iobenguane I 123 in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Preparation and Administration
- Inspect the AdreView vial for particulate matter and discoloration prior to administration. Use aseptic procedures and a radiation shielding syringe during administration. Administer the dose as an intravenous injection over 1 to 2 minutes. A subsequent injection of 0.9% sodium chloride may be used to ensure full delivery of the dose.
Monitoring
Radiation Safety
- AdreView emits radiation and must be handled with appropriate safety measures to minimize radiation exposure to clinical personnel and patients. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. AdreView dosing is based upon the radioactivity determined using a suitable calibration system immediately prior to administration.
- To minimize radiation dose to the bladder, prior to and following AdreView administration, encourage hydration to permit frequent voiding. Encourage the patient to void frequently for the first 48 hours following AdreView administration.
Thyroid Blockade
- Before administration of AdreView to patients at risk for thyroid accumulation of the drug, administer Potassium Iodide Oral Solution or Lugol's Solution (equivalent to 100 mg iodide for adults, body-weight adjusted for children) or potassium perchlorate (400 mg for adults, body-weight adjusted for children) to block uptake of iodine 123 by the patient's thyroid. Administer the blocking agent at least one hour before the dose of AdreView. Individualize thyroid blockade; for example, the blockade may not be necessary for patients who have undergone thyroidectomy or those with a very limited life expectancy.
IV Compatibility
There is limited information regarding IV Compatibility of Iobenguane I 123 in the drug label.
Overdosage
- The major manifestations of overdose relate predominantly to increased radiation exposure, with the long term risks for neoplasia.
Pharmacology
There is limited information regarding Iobenguane I 123 Pharmacology in the drug label.
Mechanism of Action
- Iobenguane is similar in structure to the antihypertensive drug guanethedine and to the neurotransmitter norepinephrine (NE). Iobenguane is, therefore, largely subject to the same uptake and accumulation pathways as NE. Iobenguane is taken up by the NE transporter in adrenergic nerve terminals and stored in the presynaptic storage vesicles. Iobenguane accumulates in adrenergically innervated tissues such as the adrenal medulla, salivary glands, heart, liver, spleen and lungs as well as tumors derived from the neural crest. By labeling iobenguane with the isotope iodine 123, it is possible to obtain scintigraphic images of the organs and tissues in which the radiopharmaceutical accumulates.
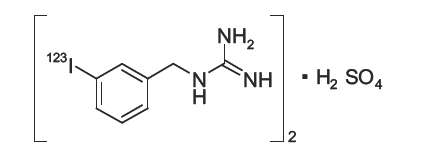
Structure
- AdreView (Iobenguane I 123 Injection) is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. Each mL contains 0.08 mg iobenguane sulfate, 74 MBq (2 mCi) of I 123 (as iobenguane sulfate I 123) at calibration date and time on the label, 23 mg sodium dihydrogen phosphate dihydrate, 2.8 mg disodium hydrogen phosphate dihydrate and 10.3 mg (1% v/v) benzyl alcohol with a pH of 5.0 – 6.5. Iobenguane sulfate I 123 is also known as I 123 meta-iodobenzlyguanidine sulfate and has the following structural formula:
Physical Characteristics
- Iodine 123 is a cyclotron-produced radionuclide that decays to Te 123 by electron capture and has a physical half-life of 13.2 hours.
[[File:Iobenguane table3.png |thumb|none|600px|This image is provided by the National Library of Medicine.]]
External Radiation
The specific gamma ray constant for iodine 123 is 1.6 R/mCi-hr at 1 cm. The first half value thickness of lead (Pb) for I 123 is 0.04 cm. The relative transmission of radiation emitted by the radionuclide that results from interposition of various thicknesses of Pb is shown in Table 4 (e.g., the use of 2.16 cm Pb will decrease the external radiation exposure by a factor of about 1,000).

Pharmacodynamics
- AdreView is a diagnostic radiopharmaceutical which contains a small quantity of iobenguane that is not expected to produce a pharmacodynamic effect. To minimize radiation dose to the thyroid gland, this organ should be blocked before dosing. Since iobenguane is excreted mainly via the kidneys, patients with severe renal insufficiency may experience increased radiation exposure and impaired imaging results. Frequent voiding should be encouraged after administration to minimize the radiation dose to the bladder.
Pharmacokinetics
- Iobenguane is rapidly cleared from the blood and accumulates in adrenergically innervated tissues. Retention is especially prolonged in highly adrenergically innervated tissues (e.g., the adrenal medulla, heart, and salivary glands).
- The majority of the iobenguane dose is excreted unaltered by the kidneys via glomerular filtration. A rapid initial clearance of circulating iobenguane is observed, followed by a slow clearance as iobenguane is released from other compartments. In patients with normal renal function, 70 to 90% of the administered dose is recovered unaltered in urine within 4 days. Iobenguane is not cleared by dialysis. Most of the remaining radioactivity recovered in the urine is in the form of the radioiodinated metabolite m-iodohippuric acid (MIHA) (typically ≤ 10%) and free radioiodide (typically ≤ 6%). The enzymatic process responsible for metabolism has not been well characterized and the pharmacologic activity of these metabolites has not been studied. Only a small amount (< 1%) of the injected dose is eliminated via the feces.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Iobenguane hemisulfate was not mutagenic in vitro in the Ames bacterial mutation assay and in the in vitro mouse lymphoma test, and was negative in the in vivo micronucleus test in rats.
- Long-term animal studies have not been conducted to evaluate AdreView's carcinogenic potential or potential effects on fertility.
Animal Toxicology and/or Pharmacology
- Iobenguane sulfate testing in dogs revealed electrocardiographic (ECG) changes after administration of 202 times the mg/m2 conversion of the maximum human dose for a 60 kg adult; the no observable effect level (NOEL) was not determined. When iobenguane was tested in a cell system stably expressing hERG-1 potassium channels, inhibition of potassium channels was not observed at an 80 μM iobenguane concentration and the IC50 was 487 μM.
Clinical Studies
Pheochromocytoma and Neuroblastoma
- The safety and efficacy of AdreView were assessed in an open-label, multicenter, multinational trial of 251 subjects with known or suspected neuroblastoma or pheochromocytoma. Diagnostic efficacy for the detection of metabolically active neuroblastoma or pheochromocytoma was determined by comparison of focal increased radionuclide uptake on planar scintigraphy at 24 ± 6 hours post-administration of AdreView against the definitive diagnosis (standard of truth). Anterior and posterior planar whole-body images, or alternatively whole-body overlapping spot images, were acquired from the head to below the knees. Additional spot images were performed as deemed appropriate at the discretion of the clinical image reviewer. SPECT imaging of the thorax and abdomen was then obtained when possible.
- Of the 251 subjects dosed with AdreView, 100 had known or suspected neuroblastoma and 151 had known or suspected pheochromocytoma. The population included 154 adults and 97 pediatric patients; the majority of adults were female (59%), the majority of pediatric subjects were male (58%). The adult subjects had a mean age of 49 years (range 17 to 88 years). The pediatric patients (56 males and 41 females) consisted of 32 infants (1 month up to 2 years of age), 62 children (2 years up to 12 years) and three adolescents (12 years up to 16 years).
- The definitive diagnosis (standard of truth) for the presence or absence of metabolically active pheochromocytoma or neuroblastoma was determined by histopathology or, when histopathology was unavailable, a composite of imaging (i.e., CT, MRI, [131I]-mIBG scintigraphy), plasma/urine catecholamine and/or catecholamine metabolite measurements, and clinical follow-up.
- A standard of truth was available for 211 subjects (127 with pheochromocytoma, 84 with neuroblastoma) and this group comprised the diagnostic efficacy population. For 93 of these subjects, the standard of truth was based solely upon histopathology. Of 211 subjects in the efficacy population, all had planar scintigraphy and 167 subjects had SPECT in addition to planar imaging. All images were assessed independently by three readers blinded to all clinical data. Table 5 summarizes the AdreView performance characteristics, by reader.

- Performance characteristics (sensitivity and specificity) of AdreView planar imaging in patients with known or suspected neuroblastoma were similar to those in patients with known or suspected pheochromocytoma. Among the selected patients who also underwent SPECT imaging, similar performance characteristics of AdreView scintigraphy were observed when SPECT plus planar imaging was compared to planar imaging alone.
Congestive Heart Failure
- The safety and efficacy of AdreView were evaluated in two open label, multicenter trials in patients with New York Heart Association (NYHA) class II or III heart failure and left ventricular ejection fraction ≤ 35%. The trials excluded subjects with an acute myocardial infarction within the prior thirty days, subjects with a functioning ventricular pacemaker as well as subjects who had received defibrillation to treat a previous arrhythmic event. Subjects underwent AdreView myocardial imaging (planar and SPECT) and continued standard clinical care; AdreView results were not used in a patient's clinical care. Mortality was assessed for up to two years after AdreView imaging and the results from the trials were analyzed using a pre-specified data integration plan.
- AdreView images in each trial were reviewed by three independent readers who assessed the H/M ratio on 3 hour 50 minute post-injection planar scintigraphy. Readers were masked to clinical information and the majority read value was used in analyses. The prognostic performance of the H/M ratio in estimating mortality was analyzed using the pre-specified 1.6 ratio cut-point to distinguish patients with higher risk from those with lower risk; other cut-points were also analyzed.
- Within the two trials, 964 patients were enrolled; 80% were men, 83% were categorized as NYHA class II and 17% as class III. The average age was 62 years (range 20 - 90 years of age). Most patients had ischemic heart disease (66%) and a history of smoking (74%). The patients were on a stable regimen of cardiovascular medications, including angiotensin converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs) (93%) and beta-blockers (92%). The range of AdreView H/M ratios in these subjects was 1.0-2.4 [mean 1.4 (± 0.2 standard deviation)].
- Within the two trials, 94 age-matched control subjects without heart disease were enrolled, 64% were men, average age was 59 years (range 29 - 82 years of age). The range of AdreView H/M ratios in these subjects was 1.1-2.4 [mean 1.8 (± 0.2 standard deviation)].
- One Year Results: By 12 months following enrollment, 50 (5%) patients had died, 61 (6%) had missing follow-up information and three patients had missing H/M ratios.
- Two Year Results: By 23 months following enrollment (the requirement for designation of two-year follow-up), 96 (10%) patients had died, 201 (21%) patients had missing follow-up information and three patients had missing H/M ratio data.
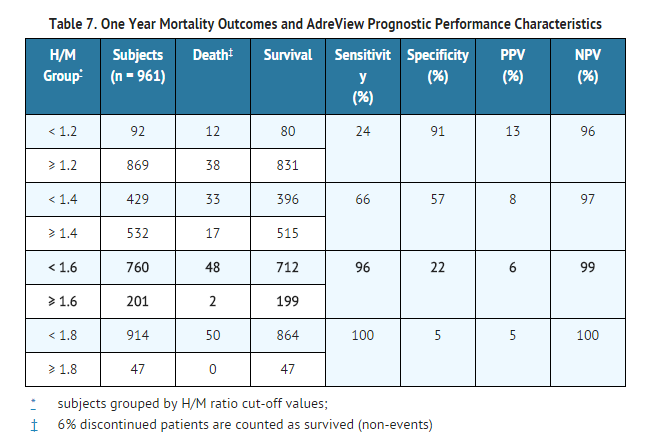
- Table 6 summarizes the mortality results by categories of H/M ratio.

H/M Ratio Prognostic Performance Characteristics:
- Follow-up mortality results were used to estimate the baseline H/M ratio prognostic performance characteristics. In these estimates, various H/M ratio "cut points" were used to group patients into those with higher versus lower H/M values, such as < 1.6 versus ≥ 1.6. The group of patients who died was examined to determine the probability of these patients having had a lower baseline H/M ratio (sensitivity). The group of patients who survived was examined to determine the probability of these patients having had a higher baseline H/M ratio (specificity). Based upon these results, the prognostic usefulness of any given H/M ratio in a patient was estimated by the positive predictive value (PPV) and the negative predictive value (NPV). The PPV is the probability of death given a lower H/M ratio; the NPV is the probability of survival given a higher H/M ratio.
- Table 7 summarizes the performance characteristics by various H/M ratio categories for one year, the time point with the most complete data. Results were similar for the two year follow-up time point analyses.
Cox Proportional Hazards Analyses:
- The association of potential risk factors with mortality for up to two years was analyzed in Cox multivariate proportional hazard modeling that included such variables as demographics, hypertension, dyslipidemia, diabetes, cardiovascular medications, smoking, and NYHA classification. The initial model included all pre-specified variables except for H/M ratio and used backward selection of variables found to be significant risk factors of all-cause mortality (p < 0.05) for inclusion in the final model. The final model consisted of the significant variables from the initial model plus the H/M ratio. In addition to age, in the final model, the H/M ratio was found to be a significant risk factor for mortality (Hazard Ratio < 0.08, p < 0.001). Left ventricular ejection fraction and brain natriuretic peptide (BNP) were not
How Supplied
- AdreView is supplied in 10 mL glass vials containing a total volume of 5 mL of solution with a total radioactivity of 370 MBq (10 mCi) at calibration time. Each vial is enclosed in a lead container of appropriate thickness.
- NDC 17156-235-01
Storage
- Store AdreView at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) . This product does not contain a preservative. Store within the original lead container or equivalent radiation shielding.
- In accordance with USP recommendations Iobenguane I 123 Injection preparations should not be used after the expiration date and time stated on the label.
Images
Drug Images
{{#ask: Page Name::Iobenguane I 123 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

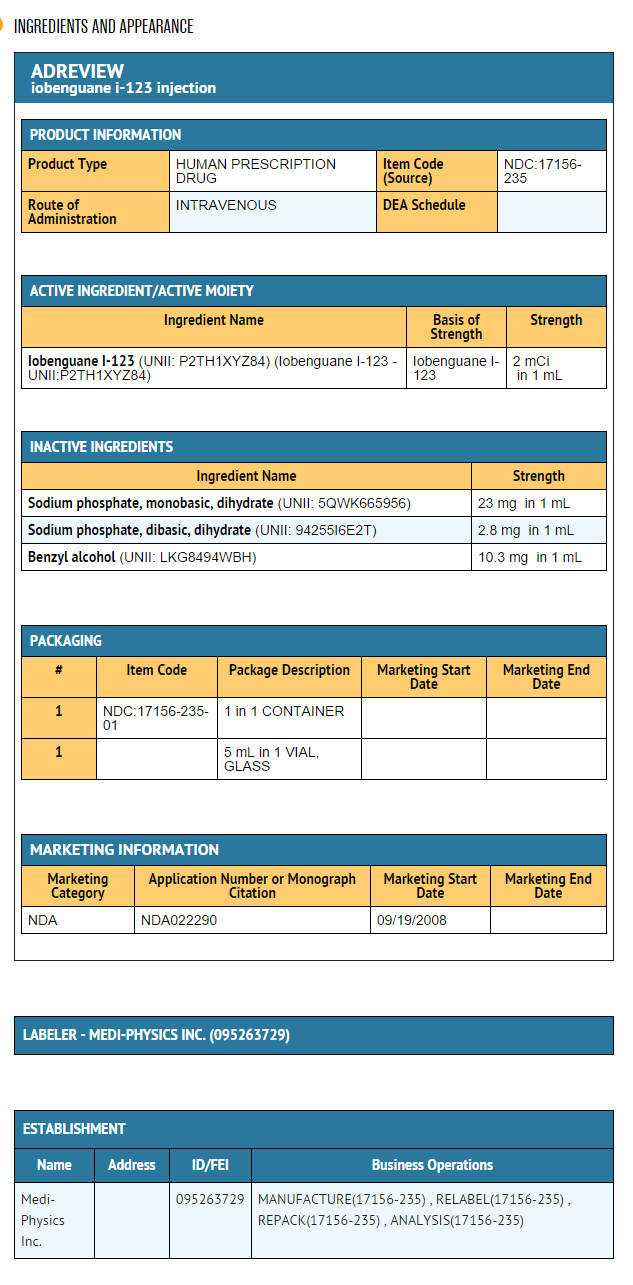
{{#ask: Label Page::Iobenguane I 123 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Instruct patients to inform their physician or healthcare provider if they:
- Are pregnant or breast feeding.
- Are sensitive to iodine, an iodine-containing contrast agent or other products that contain iodine.
- Are sensitive to Potassium Iodide Oral Solution, or Lugol's Solution.
have reduced renal function.
- Instruct patients to increase their level of hydration prior to receiving AdreView and to void frequently for the first 48 hours following AdreView administration.
Precautions with Alcohol
- Alcohol-Iobenguane I 123 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ADREVIEW®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "iobenguane i-123 injection".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Iobenguane I 123
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Iobenguane I 123 |Label Name=Iobenguane I 12311.png
}}
{{#subobject:
|Label Page=Iobenguane I 123 |Label Name=Iobenguane I 12311.png
}}