Intra-aortic balloon pump
Editors-In-Chief: Marv Slepian, M.D.; University of Arizona [1] and C. Michael Gibson M.S., M.D.
Associate-Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Synonyms and keywords: IABP, balloon pump, intraaortic balloon, intraaortic balloon pump
Overview
The Intra-aortic balloon pump (IABP) uses "counterpulsation in which a pumping unit synchronized with the patient's electrocardiogram rapidly fills a balloon in the aorta with helium or carbon dioxide in early diastole and evacuates the balloon at the onset of systole. As the balloon inflates, it raises aortic diastolic pressure, and as it deflates, it lowers aortic systolic pressure. The result is a decrease in left ventricular work and increased myocardial and peripheral perfusion."[1] IABPs are a type of assisted circulation[2]
IABP is used to decrease myocardial oxygen demand while at the same time increasing cardiac output. that used counterpulsation. By increasing cardiac output it also increases coronary blood flow and therefore myocardial oxygen delivery. It consists of a cylindrical balloon that sits in the aorta. That is, it actively deflates in systole increasing forward blood flow by reducing afterload, and actively inflates in diastole increasing blood flow to the coronary arteries. [3] [4] [5] [6] [7] [8]
Mechanism of action and physiologic benefit
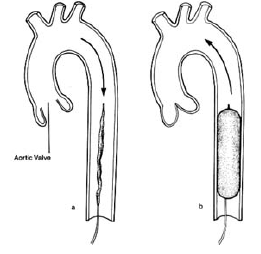
The balloon is inflated during diastole by a computer controlled, ECG linked mechanism. This controls the flow of helium from a cylinder into and out of the balloon. Helium is used because its low viscosity allows it to travel quickly through the long connecting tubes. The IABP is a polyethylene balloon mounted on a catheter, which is generally inserted into the aorta through the femoral artery in the leg. The pump is available in a wide range of sizes (2.5 cc to 50 cc) that will fit patients of any age and size. The balloon is guided into the descending aorta, and the tip of the balloon is positioned approximately 2 cm from the left subclavian artery. At the start of diastole, the balloon inflates, augmenting coronary perfusion. At the beginning of systole, the balloon deflates; this deflation occurs at the time that blood is being ejected from the left ventricle. This delation of the balloon reduces the afterload on the heart, and increases the cardiac output by as much as 40 percent. This in turn decreased the left ventricular stroke work and myocardial oxygen requirements. In this manner, the balloon supports the heart indirectly.
The IABP has two main benefits:
- The inflation of the balloon displaces blood into the ascending aorta which in turn increases the pressure there thereby increasing flow into the coronary arteries.
- The deflation of the balloon decreases end diastolic aortic pressure and consequently reduces left ventricular myocardial oxygen requirements in the following beat.
History
In 1958 Harken described a method to treat left ventricular failure by using counterpulsation or diastolic augmentation. He suggested removing a certain blood volume from the femoral artery during systole and replacing this volume rapidly during diastole. By increasing coronary perfusion pressure this concept would therefore augment cardiac output and unload the functioning heart simultaneously. This method of treatment was limited because of problems with access (need for arteriotomies of both femoral arteries), turbulence and development of massive hemolysis by the pumping apparatus. Even experimental data showed that no augmentation of coronary blood flow was obtained.
In 1961 Clauss et al. published a study regarding the concept of arterial counterpulsation.
In 1962 Moulopoulus et al. adapted the general concept of counterpulsation to the specific application of intra-aortic balloon pumping. The investigators developed an experimental prototype of the intra-aortic balloon (IAB) whose inflation and deflation times were synchronized to the cardiac cycle.
The IABP's developers thought that private insurance companies would not stand for such a radical new therapy, and approached the military, asking them to keep an eye out for IABP candidates in military hospitals. One day, the call came. A retired general was hospitalized in Walter Reed Army Medical Center. He had a history of multiple infarcts and was now end-stage. The IABP scientists were all ready to go, until they found out the general was former President Dwight D. Eisenhower. They declined the opportunity because they worried that if the IABP failed in him, it would permanently ruin the future prospects of the device.
In 1968 the IABP was first introduced into clinical practice and improved by Kantrowiz et al. In these initial years, surgical insertion and removal of the 15 french devicce was required.
In 1979, the IAB was adapted for percutaneous insertion by Bregman and Casarella, and this was followed in the early 1980s by a dual lumen, wire guided,smaller catheter device which could be placed in couple of minutes with significantly improved insertion success and reduced vascular complications.
In 1985 the prefolded IAB was introduced. Since this time further improvements in size(being reduced from 9F to 8F and requiring no sheath) and simplicity of use allow for the safe insertion of the device at the bedside by nonsurgical personnel.
Effectiveness
Clinical practice guidelines summarize indications[9].
A systematic review suggests that IABPs do not clearly improve mortality[10].
Indications & Contraindications
Intraaortic balloon counterpulsation is used in situations when the heart's own cardiac output is insufficient to meet the oxygenation demands of the body. Most commonly an IABP os placed for cardiogenic shock and the period before or after cardiac surgery but the indications for IABP includes a host of other situations.
Indications
The following situations may benefit from this device: [3] [4] [5]
- Cardiogenic shock complicating ST elevation myocardial infarction (STEMI). An analysis from the National Registry of Myocardial Infarction (NRMI) database indicates that in-hospital mortality rates are decreased at hospitals with higher rates of IABP insertion for cardiogenic shock compliacting STEMI. The raw mortality was 65.4% at hopsitals in the lowest volume tertile (3.4 IABPs/year); 54.1% at hopsitals with intermediate volume (12.7 IABPs/year); and 50.6% for hospitals with the highest volume (37.4 IABPs/yr)(P for trend <0.001). This difference in mortality would yield 150 fewer deaths per 1000 patients treated at the high IABP hospitals. Even in a multivariate analysis, hospitals with the highest IABP volume had the lowest mortality (OR=0.71, 95% CI=0.56 to 0.90), independent of baseline patient characteristics, hospital factors, treatment, and procedures such as PTCA.[11]
- Reversible mechanical defects complicating myocardial infarction, i.e. acute mitral regurgitation, papillary muscle rupture and new development of a ventricular septal defect.
- Refractory unstable angina, particularly if the patient is awaiting coronary artery bypass grafting (CABG).
- Weaning following CABG. IABP use following cardiothoracic surgery is common and is useful in supporting the hemodynamics of patients following the operative insult to the myocardium.
- Preoperative use has been suggested for high-risk patients such as those with unstable angina with stenosis greater than 70% of the left main coronary artery, and in those patients with ventricaular dysfunction with an ejection fraction less than 35%.
- Refractory ventricular failure
- Support for diagnostic coronary angiography and percutaneous coronary ıntervention in patients with high risk anatomy. Although, prophylactic use of Intra Aortic Balloon Pump may provide circulatory support during percutaneous coronary interventions in patients at high risk for procedure related morbidity and mortality, usefulness of elective IABP support in patients at high risk for complications during PCI is still controversial. Patients with pharmacologically uncontrollable chest pain, high-risk anatomic features, unprotected left main coronary artery stenosis, last remaining vessel supply, same session multivessel complex coronary interventions, coronary disease with ventricular dysfunction and severe left ventricular systolic dysfunction are likely get benefit from previous hemodynamic stabilization because even transient ischemia may produce a fatal complication. IABP therapy provides an increase in coronary artery perfusion pressure, reducing the hemodynamic consequences of reduced coronary flow during balloon inflation and other PCI modalities. Placement and institution of IABP therapy before intervening, as opposed to provisional IABP therapy only if complications occur, may prevent intraprocedural cardiac and cerebral complications.
- Ischemia related intractable ventricular arrhythmias
- Intraoperative pulsatile flow generation
- Cardiac support for non-cardiac surgery
- Myocardial contusion
- Mechanical bridge to other assist devices (such as a VAD) and heart transplant for those patients with left ventricular failure.
- Cardiac support following correction of anatomical defects in patients with congenital heart disease.
Warnings
Teleflex Medical and FDA notified healthcare professionals of the Class 1 recall for Arrow International 30cc, 40cc and 50cc Intra Aortic Balloon Pump (IAB) Catheters, a component of the Intra-Aortic Pump System which is designed to provide cardiac assist therapy to critically ill people to increase blood flow to the heart. This recall is being conducted because a fault in the connector of the pump tubing assembly may result in failure of the system to decrease ischemia and increase perfusion, leading to organ injury or infarct and may result in patient death. Prolonged exposure could also result in thrombus formation on the IAB and possible subsequent systemic or cerebral thromboembolism.
Absolute contraindications
- Moderate to severe aortic valve insufficiency
- Aortic dissection
- Severe aortoiliac occlusive disease
Relative contraindications
- Prosthetic vascular grafts in the aorta
- Aortic aneurysm
- Aortofemoral grafts
- Artificial aortic valves
- Patent ductus (counterpulsation may augment the abnormal pathway of aortic to pulmonary artery shunting)
- Bleeding diathesis
- Sepsis
- Sheathless insertion with severe obesity, scarring of the groin
Insertion of the IABP
Historical perspective
In the early years of IABP - therapy, insertion of the balloon was performed by surgical cut down to the femoral vessels. After a longitudinal incision in the groin, the femoral arteries were identified and controlled. A vascular graft was then sewn to the common femoral artery in an end-to-side fashion. The balloon was introduced into the artery via the graft and properly positioned in the thoracic aorta and the graft tightly secured to the distal portion of the balloon catheter. Finally the skin incision was closed. Removal of the balloon required a second operation.
Modern insertion techniques
Since 1979, a percutaneous placement of the IABP via the femoral artery using a modified Seldinger technique allows an easy and rapid insertion in the majority of situations. There are alternative routes for balloon insertion include subclavian, axillary or iliac arteries. After puncture of the femoral artery a J-shaped guide wire is inserted to the level of the aortic arch and then the needle is removed. The arterial puncture side is enlarged with the successive placement of an 8 to 9 Fr dilator/sheath combination. The dilator is removed and the sheath may or may not be left in place. The wrapped and deflated balloon is then inserted over the guide wire into the descending aorta approximately 1.5 to 2 cm below the left subclavian artery.
Insertion of a counterpulsation balloon without a leading guidewire under any circumstances is not recommended.
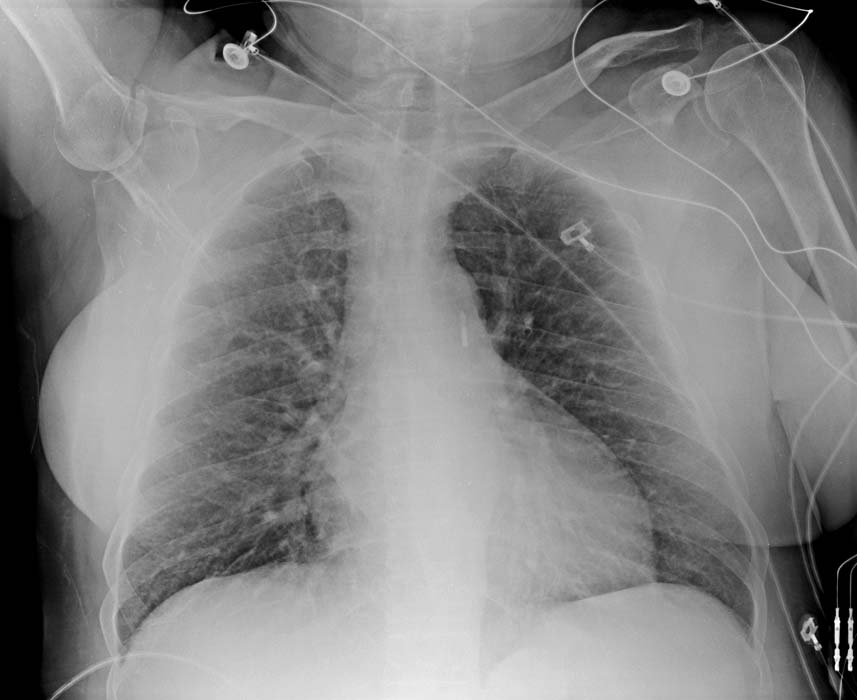
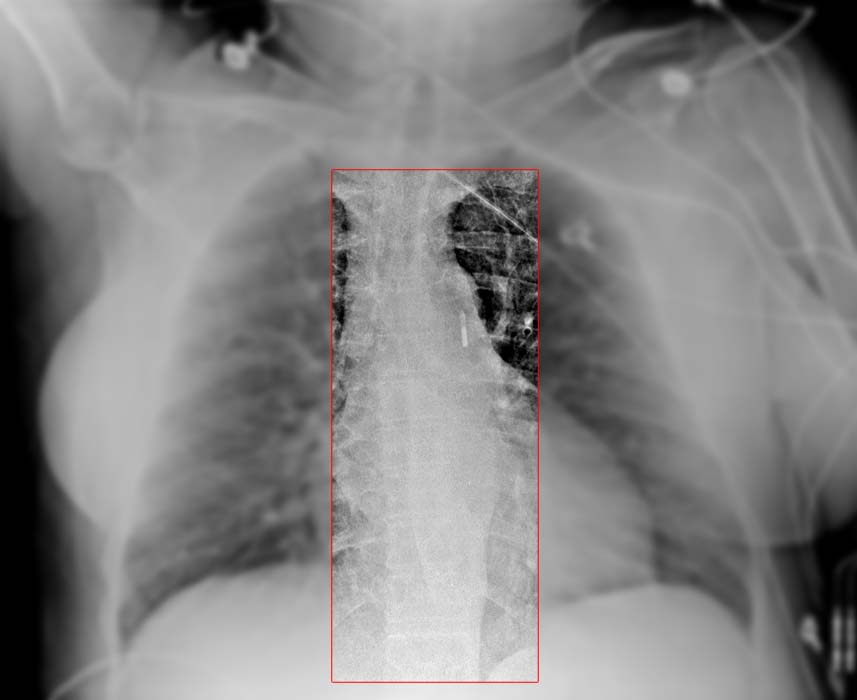
At the tip of the IABP catheter is a 1-cm metallic marker that is visible on radiographs or fluoroscopy and is used to ensure proper placement (just distal to the left subclavian artery). Once the IABP is inserted, its position should be verified on fluoroscopy. Ideally, the tip is positioned approximately 2-4 cm below the level of the aortic arch.
- If the balloon pump is too proximal in the aorta (i.e. too close to the aortic valve and head vessels), occlusion of the brachiocephalic, left carotid, or left subclavian arteries may occur.
- If the catheter is too distal, the celiac, superior mesenteric, or renal arteries may be obstructed.
The image below shows an IABP will positioned 2-4 cm below the aortic arch.
Adjunctive Pharmacotherapy to Prevent Thrombosis of the IABP
Unfractionated heparin administration (5000 U bolus and 1000 U/hr as a standard dose) is required to minimize the risk of thrombosis.
Monitoring for Complications
Continuous monitoring of the function of the IABP and vigilance for complications are essential. Continuous monitoring is required for the early detection of the following major complications of IABP insertion:
- Infection
- Thrombocytopenia: After balloon removal, the platelet count rapidly returns to normal
- Hemorrhage at the site of insertion
- Hemolysis
- Vascular obstruction with limb ischemia: regular examination of radial and dorsalis pedis arteries may be helpful for quick evaluation of peripheral arteries.
- A helium leak: should the balloon begin to leak helium, the balloon must be removed immdediately. Entrance of blood into the balloon and contact of the blood with helium in the balloon can lead to the formation of clot inside the balloon. This can dramatically increase the profile of the balloon and complicate the removal of the balloon.
Since the device is placed in the femoral artery and aorta it can cause ischemia in limbs (such as the leg) and organ beds (such as the renal and mesenteric beds). [3] [4] [5] It can also cause compartment syndrome.
Other possible complications are cerebral embolism during insertion, infection, dissection of the aorta or iliac artery, perforation of the artery and hemorrhage in the mediastinum. [3] [4] [5]
Mechanical failure of the balloon itself is also a risk which may entail vascular surgery to remove the IABP. After balloon removal there is also a risk of 'embolic shower' from micro clots that have formed on the surface of the balloon, and can lead to peripherial thrombosis, myocardial ischemia, hemodynamic decompensation, and late pseudoaneurysm. [3] [4] [5]
The success rate for percutaneous inserton of IAB is more than 90%. If signs of ischemia appear the balloon should be removed. In general, vascular injuries should be dealt with directly by surgical interventions and repair. Balloon related problems and infection require removal and / or replacement of the intra aortic balloon.
A more complete list of IABP complications include:
- Vascular
- Femoral artery perforation & dissection
- Aortic perforation & dissection
- Femoral artery thrombosis
- Peripheral embolization
- Femoral vein cannulation
- A-V fistula
- False aneurysm
- Limb ischemia
- Visceral ischemia
- Balloon related
- Perforation
- Tear
- Rupture
- Incorrect positioning
- Gas embolization
- Others
- Hemorrhage (hematomas)
- Infection
- Broken and missing balloon & catheter parts
Optimizing Timing of Inflation and Deflation of the IABP
To optimize the hemodyanmic benefits of intra aortic balloon counterpulsation, inflation and deflation must be correctly timed to the patient’s cardiac cycle. This is accomplished by either using the patient’s ECG signal, the patient’s arterial waveform or an intrinsic pump rate. The most common method of triggering the IABP is based upon the R wave of the patient’s ECG signal. The balloon inflation is then set automatically start in the middle of the T wave and to deflate prior to the ending QRS complex.
Tachyarrhythmias, cardiac pacemaker function and diminished ECG signals may impair synchronization when the ECG mode is used. In these patients, the arterial waveform may be used instead for triggering.
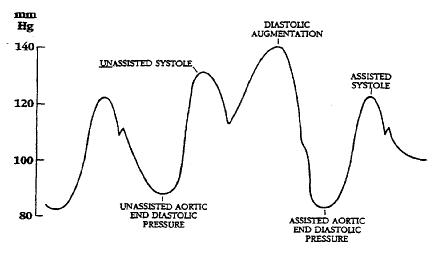
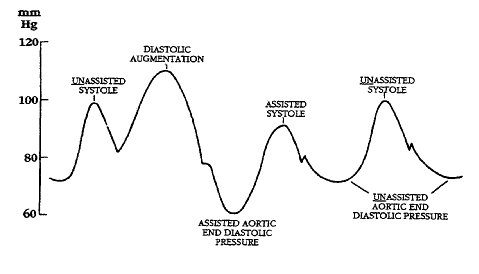
Illustrations of waveforms
-
-
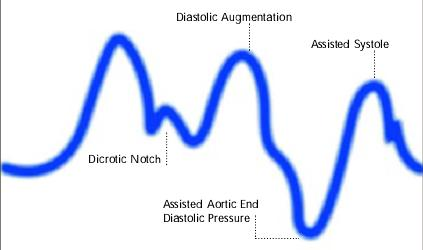
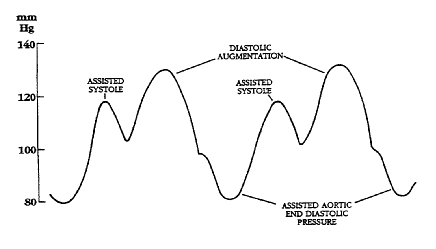
Diagram of correct intra aortic balloon timing; A= One complete cardiac cycle, B= Unassisted aortic end diastolic pressure, C= Unassisted systolic pressure, D= Diastolic augmentation, E= Reduced aortic end diastolic pressure, F= Reduced systolic pressure]]
-
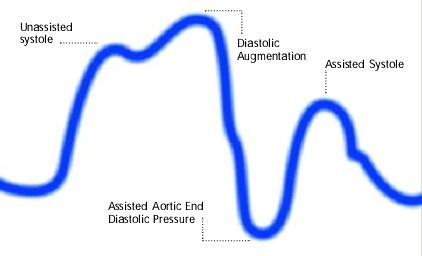
Inflation of the intra aortic balloon markedly after closure of the aortic valve (inflation of the balloon after dicrotic notch and absence of a sharp V shape)
-
Inflation of the intra aortic balloon prior to aortic valve closure (Inflation of balloon prior to dicrotic notch. Diastolic augmentation encroaches onto systole)
-
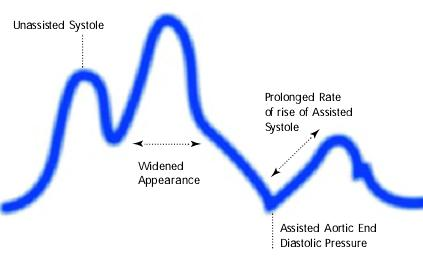
Assisted aortic end diastolic pressure may be equal to the unassisted aortic end diastolic pressure, rate of rise of assisted systole is prolonged and diastolic augmentation may appear of rise of widened
-
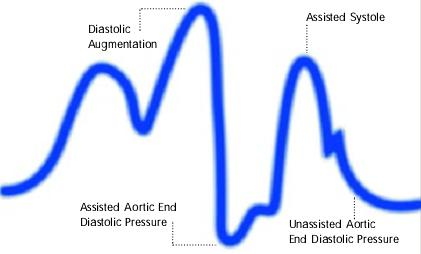
Premature deflation of the intra aortic balloon during the diastolic phase characterized as; deflation of balloon is seen as a sharp drop following diastolic augmentation, sub-optimal diastolic augmentation, assisted aortic end diastolic pressure may be equal to or less than the unassisted aortic end diastolic pressure and assisted systolic pressure may rise
Videos on IABP maintenance
ZTgJQmwETUI
{{#ev:youtube|X4gWT3u0FqQ}}
Removal of the IABP
Before removal of the IABP, the patient is slowly weaned from support by decreasing the counterpulsation rate from 1:1 to 1:2 and then 1:3. The counterpulsation rate should not be reduced below 1:8 to reduce the change of clot formation until immediately before IABP removal. In order to remove the device, aspiration or negative pressure is applied to the balloon lumen to reduce its profile. However, it should be noted that once the balloon has been inflated, its deflated profile still remains larger than the sheath lumen, and therefore it cannot completely withdrawn into sheath before removal. As a result, both the balloon and the sheath are withdrawan together while compressing the puncture site.
Anticoagulation should be discontinued 4 hours before and a partial thromboplastin time (PTT) or activated clotting time (ACT) should be checked before the balloon is removal. Pressure at the site of removal must be maintained for 30 to 60 minutes.
See also
- Cardiogenic shock
- Ventricular assist device
- Adjuncts for High Risk Percutaneous Coronary Interventions
References
- ↑ Anonymous (2024), Intra-aortic balloon pump (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Anonymous (2024), Assisted circulation (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ 3.0 3.1 3.2 3.3 3.4 Intensive Care Medicine by Irwin and Rippe
- ↑ 4.0 4.1 4.2 4.3 4.4 Intra Aortic Balloon Pump (IABP) Counterpulsation mirror with better quality by P. J Overwalder, M.D., Department of Surgery, Division of Cardiac Surgery, University Hospital Graz, The Internet Journal of Thoracic and Cardiovascular Surgery. 1999. Volume 2 Number 2.
- ↑ 5.0 5.1 5.2 5.3 5.4 Intra-aortic balloon pumping Department of Anaesthesia and Intensive Care of The Chinese University of Hong Kong
- ↑ Kreidieh I, Davies DW, Lim R, Nathan AW, Dymond DS, Banim SO. High-risk coronary angioplasty with elective intra-aortic balloon pump support. Int J Cardiol 1992, 35, 147–52
- ↑ Aguirre FV, Kern MJ, Bach R, Donohue T, Caracciolo E, Flynn MS, Wolford T. Intraaortic balloon pump support during high-risk coronary angioplasty. Cardiology 1994, 84, 175–86
- ↑ Szatmary L, Marco J, Fajadet J, Caster L, Carrie D. Intraaortic balloon counterpulsation and coronary angioplasty in high-risk coronary heart patients. Acta Med Hung 1987, 44, 189–200
- ↑ Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK; et al. (2015). "2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie D'intervention)". Catheter Cardiovasc Interv. 85 (7): E175–96. doi:10.1002/ccd.25720. PMID 25851050.
- ↑ Shi W, Wang W, Wang K, Huang W (2019). "Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention: A meta-analysis of randomized trials". Medicine (Baltimore). 98 (37): e17107. doi:10.1097/MD.0000000000017107. PMC 6750338 Check
|pmc=value (help). PMID 31517843. - ↑ Chen EW, Canto JG, Parsons LS; et al. (2003). "Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock". Circulation. 108 (8): 951–7. doi:10.1161/01.CIR.0000085068.59734.E4. PMID 12912817. Unknown parameter
|month=ignored (help)
External links
- Intra-aortic Balloon Pump (IABP) Texas Heart Institute
- Intra Aortic Balloon Pump (IABP) two videos demonstrating inflating and deflating the balloon
- Images in cardiovascular medicine The missing intra-aortic balloon pump catheter by Pasquale Totaro, Nello Degno, John Smith, Vincenzo Argano, Cardiac Surgery Department, Regional Cardiac Center, Morriston Hospital, Swansea, UK, Ital Heart J 2005; 6 (4): 361-362)
de:Intraaortale Ballonpumpe
uk:Аортальна балонна контрапульсація
![Diagram of correct intra aortic balloon timing; A= One complete cardiac cycle, B= Unassisted aortic end diastolic pressure, C= Unassisted systolic pressure, D= Diastolic augmentation, E= Reduced aortic end diastolic pressure, F= Reduced systolic pressure]]](/images/e/ee/Correct_timing.png)