Hyerthyroidism
|
Hyperthyroidism Microchapters |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Ahmed Younes M.B.B.CH [2]
Overview
Thyroid hormones are responsible for regulating the basal metabolic rate of the body. Over secretion of thyroid hormones can lead to a wide variety of syndromes depending on the cause of the hyperthyroidism. Hyperthyroidism can be due to hyperactivity of the thyroid gland itself (primary hyperthyroidism) or due to abnormalities in the pituitary gland or the hypothalamus causing irregularities in the upper control of the gland. Hyperthyroidism can also be classified according to the results of iodine uptake study into high uptake, low uptake, and high or normal uptake.
Classification
According to the origin of the abnormality
Hyperthyroidism is classified according to the origin of the lesion into[1]
Primary hyperthyroidism:
Excess thyroid production from thyroid gland
Secondary hyperthyroidism:
Excess thyroid production due to disorders of the pituitary:
Tertiary hyperthyroidism:
- Excess thyroxin production due to disorders of the hypothalamus which may be due to intracranial tumors or masses.
According to iodine uptake
Hyperthyroidism can be classified according to the results of iodine uptake test into[2]
High iodine uptake
High or normal uptake:
- Iodine caused hyperthyroidism
- Hashitoxicosis
- Germ cell tumors (choriocarcinoma in males and testicular germ cell tumors)
- Pituitary TSH producing adenoma
Low uptake:
- Subacute thyroiditis
- Hyperthyroidism due to ectopic thyroid tissue
- Factitious thyrotoxicosis
- Struma ovarii
- Painless thyroiditis
- Amiodarone induced thyroiditis-Type 1
- Amiodarone induced thyroiditis-Type 2
Differential diagnosis
| Cause of thyrotoxicosis | TSH receptor Antibodies | Thyroid US | Color flow Doppler | Radioactive iodine uptake/Scan | Other features |
|---|---|---|---|---|---|
| Graves' disease | + | Hypoechoic pattern | ↑ | ↑ | Ophthalmopathy, dermopathy, acropachy |
| Toxic nodular goiter | - | Multiple nodules | - | Hot nodules at thyroid scan | - |
| Toxic adenoma | - | Single nodule | - | Hot nodule | - |
| Subacute thyroiditis | - | Heterogeneous hypoechoic areas | Reduced/absent flow | ↓ | Neck pain, fever, and elevated inflammatory index |
| Painless thyroiditis | - | Hypoechoic pattern | Reduced/absent flow | ↓ | - |
| Amiodarone induced thyroiditis-Type 1 | - | Diffuse or nodular goiter | ↓/Normal/↑ | ↓ but higher than in Type 2 | High urinary iodine |
| Amiodarone induced thyroiditis-Type 2 | - | Normal | Absent | ↓/absent | High urinary iodine |
| Central hyperthyroidism | - | Diffuse or nodular goiter | Normal/↑ | ↑ | Inappropriately normal or high TSH |
| Trophoblastic disease | - | Diffuse or nodular goiter | Normal/↑ | ↑ | - |
| Factitious thyrotoxicosis | - | Variable | Reduced/absent flow | ↓ | ↓ serum thyroglobulin |
| Struma ovarii | - | Variable | Reduced/absent flow | ↓ | Abdominal RAIU |
| Disease | Findings | |
|---|---|---|
| Thyroiditis | Direct chemical toxicity with inflammation | Amiodarone, sunitinib, pazopanib, axitinib, and other tyrosine kinase inhibitors may also be associated with a destructive thyroiditis.[3][4] |
| Radiation thyroiditis | Patients treated with radioiodine may develop thyroid pain and tenderness 5 to 10 days later, due to radiation-induced injury and necrosis of thyroid follicular cells and associated inflammation. | |
| Drugs that interfere with the immune system | Interferon-alfa is a well known cause of thyroid abnormality. It mostly leads to the development of de novo antithyroid antibodies.[5] | |
| Lithium | Patients treated with lithium are at a high risk of developing painless thyroiditis and Graves' disease. | |
| Palpation thyroiditis | Manipulation of the thyroid gland during thyroid biopsy or neck surgery and vigorous palpation during physical examination may cause transient hyperthyroidism. | |
| Exogenous and ectopic hyperthyroidism | Factitious ingestion of thyroid hormone | The diagnosis is based upon the clinical features, laboratory findings, and 24-hour radioiodine uptake.[6] |
| Acute hyperthyroidism from a levothyroxine overdose | The diagnosis is based upon the clinical features, laboratory findings, and 24-hour radioiodine uptake.[7] | |
| Struma ovarii | Functioning thyroid tissue is present in an ovarian neoplasm. | |
| Functional thyroid cancer metastases | Large bony metastases from widely metastatic follicular thyroid cancer cause symptomatic hyperthyroidism. | |
| Hashitoxicosis | It is an autoimmune thyroid disease that initially presents with hyperthyroidism and a high radioiodine uptake caused by TSH-receptor antibodies similar to Graves' disease. It is then followed by the development of hypothyroidism due to the infiltration of thyroid gland with lymphocytes and the resultant autoimmune-mediated destruction of thyroid tissue, similar to chronic lymphocytic thyroiditis.[8] | |
| Toxic adenoma and toxic multinodular goiter | Toxic adenoma and toxic multinodular goiter are results of focal/diffuse hyperplasia of thyroid follicular cells independent of TSH regulation. Findings of single or multiple nodules are seen on physical examination or thyroid scan.[9] | |
| Iodine-induced hyperthyroidism | It is uncommon but can develop after an iodine load, such as administration of contrast agents used for angiography or computed tomography (CT), or iodine-rich drugs such as amiodarone. | |
| Trophoblastic disease and germ cell tumors | Thyroid-stimulating hormone and HCG have a common alpha-subunit and a beta-subunit with considerable homology. As a result, HCG has weak thyroid-stimulating activity and high titer HCG may mimic hyperthyroidism.[10] | |
Pathophysiology
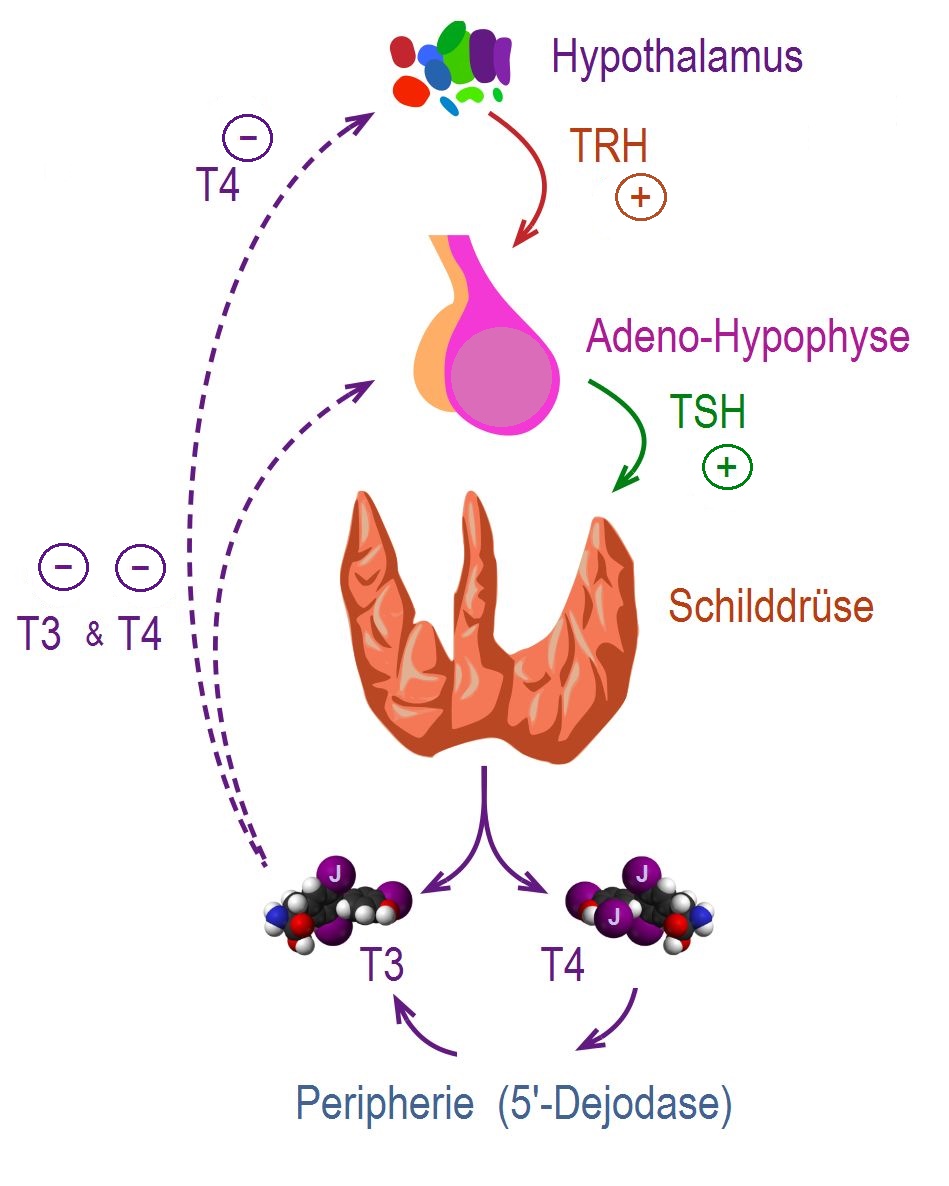 |
- Thyroid hormones (T3 and T4) are regulating basal metabolic rate, influence oxygen consumption by tissues. They are crucial for normal development of the brain and growth of the body, especially in prepubertal period.[11]
- Secretion of thyroid hormones follows upper control from the hypothalamus and the pituitary. Thyroid releasing hormone (TRH) acts on thyrotropes releasing cells in the pituitary causing them to release thyroid stimulating hormone (TSH).
- TSH acts on thyroid gland by binding to specific membrane receptors and activating an intracellular pathway involving cAMP that ends in formation and secretion of thyroid hormones.
- Iodine is essential for the synthesis of thyroid hormones. The daily iodide need is about 100mcg / day. Iodide is uptaken through a special Na/I transporter found in the membrane of thyroid follicular cell. After uptaking iodide, it goes through a series of organic reactions ending in the formation of the two forms of thyroid hormones: T3 and T4. T3 and T4 remain stored in the thyroglobulin of the follicles and are released in response to further stimulation by TSH to the thyroid follicles.
- While T3 is 3 to 5 times more potent than T4, it represents only one fourth of the total hormone secretion. T3 is thought to be the biologically active form of the of the two forms of the hormone. Most of the circulating T3 is due to peripheral conversion of T4 in the liver and peripheral tissues while only a small percentage is secreted directly from the thyroid gland itself.
- T3 and T4 act on nuclear receptors (DNA binding proteins) and cause the regulate the transcription of many proteins to regulate the metabolic rate of the body.
- The higher regulation of thyroxin secretion follows the negative feedback role, meaning that high levels of T3 and T4 will suppress TRH and TSH secretion and vice versa (Low levels of thyroxins will stimulate TRH and TSH secretion). This is useful in diagnosing the cause of hyperthyroidism (in secondary hyperthyroidism where the pituitary or the hypothalamus are the sources of the disease. TSH will be high, while in primary hyperthyroidism where the gland is the source of the excess hormones, TSH will be low).
- In graves' disease, the most common cause of hyperthyroidism. The disorder lies in the secretion of thyroid stimulating antibodies (TSI) that work on thyroid follicular cells causing an excessive uncontrolled release of the thyroxins. TSI responsible for many other aspects of the disease such as ophthalmopathy and the skin manifestations. This is thought to be due to the epitopic similarity between antigens on the surface of these cells and the thyroid receptors.[12]
- Toxic nodular goiter involves the growth of a various number of nodules (ranging from one to tens). These nodules either bleed and undergo degeneration and fibrosis followed by calcification or they might have autonomous activity producing excess thyroxin.
- The majority of circulating T3 and T4 are bound to plasma proteins and thus not active (T4 is mostly bound to thyroxine binding globulin and T3 is mostly bound to transthyretin). Conditions that impair the production of thyroid binding globulins (such as pregnancy, liver failure, and certain drug administration) cause a change in the total serum thyroxins but the free T3 and T4 remain normal and the patient remains euthyroid (this carries only laboratory significance).[13]
References
- ↑ Monaco F (2003). "Classification of thyroid diseases: suggestions for a revision". J. Clin. Endocrinol. Metab. 88 (4): 1428–32. doi:10.1210/jc.2002-021260. PMID 12679417.
- ↑ [+http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(05)72981-0/abstract "Thyroid disease classification - The Lancet"] Check
|url=value (help). - ↑ Lambert M, Unger J, De Nayer P, Brohet C, Gangji D (1990). "Amiodarone-induced thyrotoxicosis suggestive of thyroid damage". J. Endocrinol. Invest. 13 (6): 527–30. PMID 2258582.
- ↑ Ahmadieh H, Salti I (2013). "Tyrosine kinase inhibitors induced thyroid dysfunction: a review of its incidence, pathophysiology, clinical relevance, and treatment". Biomed Res Int. 2013: 725410. doi:10.1155/2013/725410. PMC 3824811. PMID 24282820.
- ↑ Vialettes B, Guillerand MA, Viens P, Stoppa AM, Baume D, Sauvan R, Pasquier J, San Marco M, Olive D, Maraninchi D (1993). "Incidence rate and risk factors for thyroid dysfunction during recombinant interleukin-2 therapy in advanced malignancies". Acta Endocrinol. 129 (1): 31–8. PMID 8351956.
- ↑ Cohen JH, Ingbar SH, Braverman LE (1989). "Thyrotoxicosis due to ingestion of excess thyroid hormone". Endocr. Rev. 10 (2): 113–24. doi:10.1210/edrv-10-2-113. PMID 2666114.
- ↑ Jha S, Waghdhare S, Reddi R, Bhattacharya P (2012). "Thyroid storm due to inappropriate administration of a compounded thyroid hormone preparation successfully treated with plasmapheresis". Thyroid. 22 (12): 1283–6. doi:10.1089/thy.2011.0353. PMID 23067331.
- ↑ Fatourechi V, McConahey WM, Woolner LB (1971). "Hyperthyroidism associated with histologic Hashimoto's thyroiditis". Mayo Clin. Proc. 46 (10): 682–9. PMID 5171000.
- ↑ Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G (1991). "High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves' disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland". J. Intern. Med. 229 (5): 415–20. PMID 2040867.
- ↑ Oosting SF, de Haas EC, Links TP, de Bruin D, Sluiter WJ, de Jong IJ, Hoekstra HJ, Sleijfer DT, Gietema JA (2010). "Prevalence of paraneoplastic hyperthyroidism in patients with metastatic non-seminomatous germ-cell tumors". Ann. Oncol. 21 (1): 104–8. doi:10.1093/annonc/mdp265. PMID 19605510.
- ↑ Kirsten D (2000). "The thyroid gland: physiology and pathophysiology". Neonatal Netw. 19 (8): 11–26. doi:10.1891/0730-0832.19.8.11. PMID 11949270.
- ↑ ADAMS DD (1965). "PATHOGENESIS OF THE HYPERTHYROIDISM OF GRAVES'S DISEASE". Br Med J. 1 (5441): 1015–9. PMC 2166943. PMID 14262190.
- ↑ Chopra IJ, Solomon DH (1983). "Pathogenesis of hyperthyroidism". Annu. Rev. Med. 34: 267–81. doi:10.1146/annurev.me.34.020183.001411. PMID 6134495.