Gadoterate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
See full prescribing information for complete Boxed Warning.
NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
The risk for NSF appears highest among patients with: Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or Acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing (5.1). For patients at highest risk for NSF, do not exceed the recommended DOTAREM dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration |
Overview
Gadoterate is a Diagnostic Agent that is FDA approved for the diagnosis of areas with disruption of the Blood brain barrier (BBB) and/or abnormal vascularity in brain (intracranial), spine and associated tissues with magnetic resonance imaging (MRI). There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, headache, injection site pain, injection site coldness, and burning sensation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- DOTAREM is a gadolinium-based contrast agent indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (2 years of age and older) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.
Dosage
Dosing Guidelines
- For adult and pediatric patients (2 years and older), the recommended dose of DOTAREM is 0.2 mL/kg (0.1 mmol/kg) body weight administered as an intravenous bolus injection, manually or by power injector, at a flow rate of approximately 2 mL/second for adults and 1 - 2 mL/second for pediatric patients. Table 1 provides weight-adjusted dose volumes.
- To ensure complete injection of DOTAREM the injection may be followed by normal saline flush. Contrast MRI can begin immediately following DOTAREM injection.
Drug Handling
- Visually inspect DOTAREM for particulate matter prior to administration. Do not use the solution if particulate matter is present or if the container appears damaged. DOTAREM should be a clear, colorless to yellow solution. Do not mix with other drugs or parenteral nutrition. Discard any unused portions of the drug.
- When DOTAREM is to be injected using plastic disposable syringes, the contrast medium should be drawn into the syringe and used immediately.
DOSAGE FORMS AND STRENGTHS
- DOTAREM 0.5 mmol/mL is a sterile, clear, colorless to yellow, aqueous solution for intravenous injection containing 376.9 mg/mL gadoterate meglumine and is available in vials and pre-filled syringes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadoterate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadoterate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- DOTAREM is a gadolinium-based contrast agent indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (2 years of age and older) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.
Dosage
Dosing Guidelines
- For adult and pediatric patients (2 years and older), the recommended dose of DOTAREM is 0.2 mL/kg (0.1 mmol/kg) body weight administered as an intravenous bolus injection, manually or by power injector, at a flow rate of approximately 2 mL/second for adults and 1 - 2 mL/second for pediatric patients. Table 1 provides weight-adjusted dose volumes.
To ensure complete injection of DOTAREM the injection may be followed by normal saline flush. Contrast MRI can begin immediately following DOTAREM injection.
Drug Handling
- Visually inspect DOTAREM for particulate matter prior to administration. Do not use the solution if particulate matter is present or if the container appears damaged. DOTAREM should be a clear, colorless to yellow solution. Do not mix with other drugs or parenteral nutrition. Discard any unused portions of the drug.
- When DOTAREM is to be injected using plastic disposable syringes, the contrast medium should be drawn into the syringe and used immediately.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadoterate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadoterate in pediatric patients.
Contraindications
- History of clinically important hypersensitivity reactions to DOTAREM
Warnings
|
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
See full prescribing information for complete Boxed Warning.
NEPHROGENIC SYSTEMIC FIBROSIS (NSF):
The risk for NSF appears highest among patients with: Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or Acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing (5.1). For patients at highest risk for NSF, do not exceed the recommended DOTAREM dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration |
Nephrogenic Systemic Fibrosis
- Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 - 59 mL/min/1.73 m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60 - 89 mL/min/1.73 m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following DOTAREM administration to Guerbet LLC (1-877-729-6679) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
- Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
- Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended DOTAREM dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination. The usefulness of hemodialysis in the prevention of NSF is unknown .
Hypersensitivity Reactions
- Anaphylactic and anaphylactoid reactions have been reported with DOTAREM, involving cardiovascular, respiratory, and/or cutaneous manifestations. Some patients experienced circulatory collapse and died. In most cases, initial symptoms occurred within minutes of DOTAREM administration and resolved with prompt emergency treatment.
- Before DOTAREM administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to DOTAREM.
- Administer DOTAREM only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation.
- During and following DOTAREM administration, observe patients for signs and symptoms of hypersensitivity reactions.
Acute Kidney Injury
- In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent; administer the lowest dose necessary for adequate imaging. Screen all patients for renal impairment by obtaining a history and/or laboratory tests. Consider follow-up renal function assessments for patients with a history of renal dysfunction.
Extravasation and Injection Site Reactions
- Ensure catheter and venous patency before the injection of DOTAREM. Extravasation into tissues during DOTAREM administration may result in tissue irritation
Adverse Reactions
Clinical Trials Experience
- GBCAs have been associated with a risk for NSF. NSF has not been reported in patients with a clear history of exposure to DOTAREM alone.
- Hypersensitivity reactions and acute kidney injury are described in other sections of the labeling.
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The data described below reflect DOTAREM exposure in 2813 patients, representing 2672 adults and 141 pediatric patients. Overall, 55% of the patients were men. In clinical trials where ethnicity was recorded the ethnic distribution was 74% Caucasian, 12% Asian, 4% Black, and 10% others. The average age was 53 years (range from 0.1 to 97 years).
- Overall, 3.9% of patients reported at least one adverse reaction, primarily occurring immediately or several days following DOTAREM administration. Most adverse reactions were mild or moderate in severity and transient in nature.
- Table 2 lists adverse reactions that occurred in ≥ 0.2% patients who received DOTAREM.
- Adverse reactions that occurred with a frequency < 0.2% in patients who received DOTAREM include: feeling cold, rash, somnolence, fatigue, dizziness, vomiting, pruritus, paresthesia, dysgeusia, pain in extremity, anxiety, hypertension, palpitations, oropharyngeal discomfort, serum creatinine increased and injection site reactions, including site inflammation, extravasation, pruritus, and warmth.
Adverse Reactions in Pediatric Patients
- During clinical trials, 141 pediatric patients (7 aged < 24 months, 33 aged 2 - 5 years, 58 aged 6 - 11 years and 43 aged 12 - 17) received DOTAREM. Overall, 6 pediatric patients (4.3%) reported at least one adverse reaction following DOTAREM administration. The most frequently reported adverse reaction was headache (1.5%). Most adverse events were mild in severity and transient in nature, and all patients recovered without treatment.
Postmarketing Experience
- The following additional adverse reactions have been identified during postmarketing use of DOTAREM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
- DOTAREM does not interfere with serum and plasma calcium measurements determined by colorimetric assays. Specific drug interaction studies with DOTAREM have not been conducted.
Use in Specific Populations
Pregnancy
- Risk Summary
- There are no adequate and well-controlled studies with DOTAREM conducted in pregnant women. Limited published human data on exposure to other GBCAs during pregnancy did not show adverse effects in exposed neonates. No effects on embryo fetal development were observed in rats or rabbits at doses up to 10 mmol/kg/day in rats or 3 mmol/kg/day in rabbits. The doses in rats and rabbits were respectively 16 and 10 times the recommended human dose based on body surface area. DOTAREM should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Human Data
- While it is unknown if DOTAREM crosses the human placenta, other GBCAs do cross the placenta in humans and result in fetal exposure.
Animal Data
- Reproductive and developmental toxicity studies were conducted with gadoterate meglumine in rats and rabbits. Gadoterate meglumine was administered intravenously in doses of 0, 2, 4 and 10 mmol/kg/day (or 3.2, 6.5 and 16.2 times the recommended human dose based on body surface area) to female rats for 14 days before mating throughout the mating period and until gestation day (GD) 17. Pregnant rabbits were intravenously administered gadoterate meglumine at the dose levels of 0, 1, 3 and 7 mmol/kg/day (or 3.3, 10 and 23 times the human doses based on body surface area) from GD6 to GD19. No effects on embryo fetal development were observed in rats or rabbits at doses up to 10 mmol/kg/day in rats or 3 mmol/kg/day in rabbits. Maternal toxicity was observed in rats at 10 mmol/kg/day (or 16 times the human dose based on body surface area) and in rabbits at 7 mmol/kg/day (23 times the human dose based on body surface area).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Gadoterate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Gadoterate during labor and delivery.
Nursing Mothers
- It is not known whether DOTAREM is excreted in human milk. Limited case reports on use of GBCAs in nursing mothers indicate that 0.01 to 0.04% of the maternal gadolinium dose is excreted in human breast milk. Because many drugs are excreted in human milk, exercise caution when DOTAREM is administered to a nursing woman. Nonclinical data show that gadoterate meglumine is excreted into breast milk in very small amounts (<0.1% of the dose intravenously administered) and the absorption via the gastrointestinal tract is poor.
Pediatric Use
- The safety and efficacy of DOTAREM at a single dose of 0.1 mmol/kg have been established in pediatric patients from 2 to 17 years of age. No dosage adjustment according to age is necessary in this population. The safety and efficacy of DOTAREM have not been established in pediatric patients below 2 years of age. GFR does not reach adult levels until 1 year of age
Geriatic Use
- In clinical studies of DOTAREM, 900 patients were 65 years of age and over, and 312 patients were 75 years of age and over. No overall differences in safety or efficacy were observed between these subjects and younger subjects. In general, use of DOTAREM in elderly patients should be cautious, reflecting the greater frequency of impaired renal function and concomitant disease or other drug therapy. No age-related dosage adjustment is necessary.
Gender
There is no FDA guidance on the use of Gadoterate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gadoterate with respect to specific racial populations.
Renal Impairment
- No DOTAREM dosage adjustment is recommended for patients with renal impairment. Gadoterate meglumine can be removed from the body by hemodialysis
Hepatic Impairment
There is no FDA guidance on the use of Gadoterate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gadoterate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gadoterate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing
IV Compatibility
There is limited information regarding IV Compatibility of Gadoterate in the drug label.
Overdosage
- DOTAREM administered to healthy volunteers and to patients at cumulative doses up to 0.3 mmol/kg was tolerated in a manner similar to lower doses. Adverse reactions to overdosage with DOTAREM have not been reported. Gadoterate meglumine can be removed from the body by hemodialysis
Pharmacology
| |
Gadoterate
| |
| Systematic (IUPAC) name | |
| gadolinium(+3) cation; 2-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetate | |
| Identifiers | |
| CAS number | |
| ATC code | V08 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 558.64 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Gadoterate is a paramagnetic molecule that develops a magnetic moment when placed in a magnetic field. The magnetic moment enhances the relaxation rates of water protons in its vicinity, leading to an increase in signal intensity (brightness) of tissues.
- In magnetic resonance imaging (MRI), visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occurs with:
- differences in proton density
- differences of the spin-lattice or longitudinal relaxation times (T1)
- differences in the spin-spin or transverse relaxation time (T2)
- When placed in a magnetic field, gadoterate shortens the T1 and T2 relaxation times in target tissues. At recommended doses, the effect is observed with greatest sensitivity in the T1-weighted sequences.
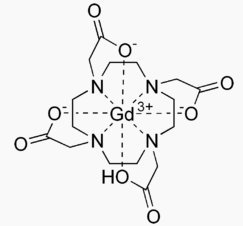
Structure
- DOTAREM (gadoterate meglumine) is a paramagnetic macrocyclic ionic contrast agent administered for magnetic resonance imaging. The chemical name for gadoterate meglumine is D-glucitol, 1-deoxy-1-(methylamino)-,[1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraaceto(4-)-.kappa.N1, .kappa.N4, .kappa.N7, .kappa.N10, .kappa.O1, .kappa.O4, .kappa.O7, .kappa.O10]gadolinate(1-)(1:1); it has a formula weight of 753.9 g/mol and empirical formula of C23H42O13N5Gd (anhydrous basis).
- The structural formula of gadoterate meglumine in solution is as follows:
- DOTAREM Injection is a sterile, nonpyrogenic, clear, colorless to yellow, aqueous solution of 0.5 mmol/mL of gadoterate meglumine. No preservative is added. Each mL of DOTAREM contains 376.9 mg of gadoterate meglumine, 0.25 mg of DOTA and water for injection. DOTAREM has a pH of 6.5 to 8.0.
- The main physiochemical properties of DOTAREM are provided below:
- The thermodynamic stability constants for gadoterate (log Ktherm and log Kcond at pH 7.4) are 25.6 and 19.3, respectively.
Pharmacodynamics
- Gadoterate affects proton relaxation times and consequently the MR signal, and the contrast obtained is characterized by the relaxivity of the gadoterate molecule. The relaxivity values for gadoterate are similar across the spectrum of magnetic field strengths used in clinical MRI (0.2 - 1.5 T).
- Gadoterate does not cross the intact blood-brain barrier and, therefore, does not enhance normal brain or lesions that have a normal blood-brain barrier, e.g. cysts, mature post-operative scars. However, disruption of the blood-brain barrier or abnormal vascularity allows distribution of gadoterate in lesions such as neoplasms, abscesses, and infarcts.
Pharmacokinetics
- The pharmacokinetics of total gadolinium following an intravenously administered 0.1 mmol/kg dose of DOTAREM in normal subjects conform to a one-compartment open-model with a mean elimination half-life (reported as mean ± SD) of about 1.4 ± 0.2 hr and 2.0 ± 0.7 hr in female and male subjects, respectively. Similar pharmacokinetic profile and elimination half-life values were observed after intravenous injection of 0.1 mmol/kg of DOTAREM followed 20 minutes later by a second injection of 0.2 mmol/kg (1.7 ± 0.3 hr and 1.9 ± 0.2 hr in female and male subjects, respectively).
Distribution
- The volume of distribution at steady state of total gadolinium in normal subjects is 179 ± 26 and 211 ± 35 mL/kg in female and male subjects respectively, roughly equivalent to that of extracellular water.
- Gadoterate does not undergo protein binding in vitro. The extent of blood cell partitioning of gadoterate is not known.
Metabolism
- Gadoterate is not known to be metabolized.
Elimination
- Following a 0.1 mmol/kg dose of DOTAREM, total gadolinium is excreted primarily in the urine with 72.9 ± 17.0 % and 85.4 ± 9.7 % (mean ± SD) eliminated within 48 hours, in female and male subjects, respectively. Similar values were achieved after a cumulative dose of 0.3 mmol/kg (0.1 + 0.2 mmol/kg, 20 minutes later), with 85.5 ± 13.2 % and 92.0 ± 12.0 % recovered in urine within 48 hrs in female and male subjects respectively.
- In healthy subjects, the renal and total clearance rates of total gadolinium are comparable (1.27 ± 0.32 and 1.74 ± 0.12 mL/min/kg in females; and 1.40 ± 0.31 and 1.64 ± 0.35 mL/min/kg in males, respectively) indicating that the drug is primarily cleared through the kidneys. Within the studied dose range (0.1 to 0.3 mmol/kg), the kinetics of total gadolinium appear to be linear.
Special Populations
Renal Impairment
- A single intravenous dose of 0.1 mmol/kg of DOTAREM was administered to 8 patients (5 men and 3 women) with impaired renal function (mean serum creatinine of 498 ± 98 µmol/L in the 10 - 30 mL/min creatinine clearance group and 192 ± 62 µmol/L in the 30 - 60 mL/min creatinine clearance group). Renal impairment delayed the elimination of total gadolinium. Total clearance decreased as a function of the degree of renal impairment. The distribution volume was unaffected by the severity of renal impairment (Table 5). No changes in renal function test parameters were observed after DOTAREM injection. The mean cumulative urinary excretion of total gadolinium was approximately 76.9 ± 4.5% in 48 hrs in patients with moderate renal impairment, 68.4 ± 3.5% in 72 hrs in patients with severe renal impairment and 93.3 ± 4.7% in 24 hrs for subjects with normal renal function.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term animal studies have not been performed to evaluate the carcinogenic potential of gadoterate meglumine.
- Gadoterate meglumine did not demonstrate mutagenic potential in in vitro bacterial reverse mutation assays (Ames test) using Salmonella typhimurium, in an in vitro chromosome aberration assay in Chinese hamster ovary cells, in an in vitro gene mutation assay in Chinese hamster lung cells, nor in an in vivo mouse micronucleus assay.
- No impairment of male or female fertility and reproductive performance was observed in rats after intravenous administration of gadoterate meglumine at the maximum tested dose of 10 mmol/kg/day (16 times the maximum human dose based on surface area), given during more than 9 weeks in males and more than 4 weeks in females. Sperm counts and sperm motility were not adversely affected by treatment with the drug.
Animal Toxicology and/or Pharmacology
- Local intolerance reactions, including moderate irritation associated with infiltration of inflammatory cells were observed after perivenous injection in rabbits suggesting the possibility of local irritation if the contrast medium leaks around the veins in a clinical setting
Clinical Studies
- Efficacy and safety of DOTAREM were evaluated in a multi-center clinical trial (Study A) that enrolled 364 adult and 38 pediatric patients (aged ≥ 2 years) with known or suspected CNS lesions. Adults were randomized 2 to 1 to receive either DOTAREM or gadopentetate dimeglumine, each administered at a dose of 0.1 mmol/kg. All pediatric patients received DOTAREM, also at a dose of 0.1 mmol/kg. In the trial, patients first underwent a baseline (pre-contrast) MRI examination followed by the assigned GBCA administration and a post-contrast MR examination. The images (pre-contrast, post-contrast and “paired pre- and post-contrast”) were interpreted by three independent off-site readers blinded to clinical information. The primary efficacy analysis compared three patient-level visualization scores (paired images) to baseline MRI (pre-contrast images) for adults who received DOTAREM. The three primary visualization components were: contrast enhancement, border delineation and internal morphology. For each of these components there was a pre-defined scoring scale. Lesion counting (up to five per patient) was also reflected within each component’s patient-level visualization score.
- Among the adult patients, 245 received DOTAREM and their data comprised the primary efficacy population. There were 114 (47%) men and 131 (53%) women with a mean age of 53 years (range 18 to 85 years), the racial and ethnic representations were 84% Caucasian, 11% Asian, 4% Black, and 1% other.
- Table 6 displays a comparison of paired images (pre-and post-contrast) to pre-contrast images with respect to the proportion of patients who had paired image scores that were greater “better”, or same/worse “not better” than the pre-contrast scores and with respect to the difference in the mean patient level visualization score. Across the three readers 56% to 94% of patients had improved lesion visualization for paired images compared to pre-contrast images. DOTAREM provided a statistically significant improvement for all three primary visualization components. More lesions were seen on the paired images than the pre-contrast images.
- In secondary analyses, post-contrast images were improved in comparison to pre-contrast images. DOTAREM lesion visualization scores were similar to those for gadopentetate dimeglumine. DOTAREM imaging results in the pediatric patients were also similar to those seen in adults.
- In a second clinical trial (Study B), MR images were reread from 150 adult patients with known CNS lesions who had participated in previously conducted clinical trial. DOTAREM administration and image interpretation was performed in the same manner as in Study A. Similar to Study A, this trial also demonstrated improved lesion visualization with DOTAREM.
How Supplied
- DOTAREM Injection is a clear, colorless to yellow solution containing 0.5 mmol/mL of gadoterate meglumine. It is supplied in vials and prefilled syringes.
- DOTAREM Injection is supplied in 10 mL vials containing 10 mL of solution, in 20 mL vials containing 15 mL or 20 mL of solution.
- Each single dose vial is closed with a rubber stopper and sealed with an aluminum cap and the contents are sterile. Vials are individually packaged in a shrink wrapped package of 10, in the following configurations:
- 10 mL in glass vial (NDC 67684-2000-1)
- 15 mL in glass vial (NDC 67684-2000-2)
- 20 mL in glass vial (NDC 67684-2000-3)
- DOTAREM Injection is supplied in 10 mL pre-filled syringes containing 10 mL of solution and 20 mL pre-filled syringes containing 15 mL or 20 mL of solution.
Each syringe is sealed with rubber closures and the contents are sterile. Syringes, including plunger rod, are packaged in a shrink wrapped package of 5, in the following configurations:
- 10 mL in glass pre-filled syringe (NDC 67684-2000-5)
- 15 mL in glass pre-filled syringe (NDC 67684-2000-6)
- 20 mL in glass pre-filled syringe (NDC 67684-2000-7)
Storage
- Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP, Controlled Room Temperature (CRT)].
- Pre-filled syringes must not be frozen. Frozen syringes should be discarded.
- Should solidification occur in the vial because of exposure to the cold, DOTAREM should be brought to room temperature before use. If allowed to stand at room temperature for a minimum of 90 minutes, DOTAREM should return to a clear, colorless to yellow solution. Before use, examine the product to assure that all solids are redissolved and that the container and closure have not been damaged. Should solids persist, discard the vial.
- Directions for Use of the DOTAREM (gadoterate meglumine) Injection glass pre-filled syringe:
- Screw the threaded tip of the plunger rod clockwise into the cartridge plunger and push forward a few millimeters to break any friction between the cartridge plunger and syringe barrel.
- Holding the syringe vertically so the rubber cap is pointed upward, aseptically remove the rubber cap from the tip of the syringe and attach either a sterile, disposable needle or compatible needleless luer lock tubing set using a push-twist action. At this point, the tubing set is not attached to a patient’s intravenous connection.
- If using a needleless luer lock tubing set, check the connection between the syringe and the tubing as the fluid flows. Ensure that the connection is successful before administration of DOTAREM Injection.
- If using a needle, hold the syringe vertically and push plunger forward until all of the air is evacuated and fluid either appears at the tip of the needle or the tubing is filled. Following the usual venous blood aspiration procedure, complete the DOTAREM injection.
- To ensure complete delivery of the contrast medium, the injection may be followed by a normal saline flush.
Properly dispose of the syringe and any other materials used.
Images
Drug Images
{{#ask: Page Name::Gadoterate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL PACKAGE DISPLAY
Ingredients and Appearance
{{#ask: Label Page::Gadoterate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Nephrogenic Systemic Fibrosis
- Instruct patients to inform their healthcare provider if they:
- have a history of kidney disease, or
- have recently received a GBCA.
- GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. To counsel patients at risk for NSF:
- Describe the clinical manifestations of NSF.
- Describe procedures to screen for the detection of renal impairment.
- Instruct the patients to contact their physician if they develop signs or symptoms of NSF following DOTAREM administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
Common Adverse Reactions
- Inform patients that they may experience:
- Reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site.
- Side effects of headache, nausea, abnormal taste and feeling hot.
General Precautions
- Instruct patients receiving DOTAREM to inform their physician if they:
- Are pregnant or breastfeeding.
- Have a history of allergic reaction to contrast media, bronchial asthma or allergy.
- Are taking any medications.
Precautions with Alcohol
- Alcohol-Gadoterate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dotarem®[1]
Look-Alike Drug Names
There is limited information regarding look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.









