Fosfomycin adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
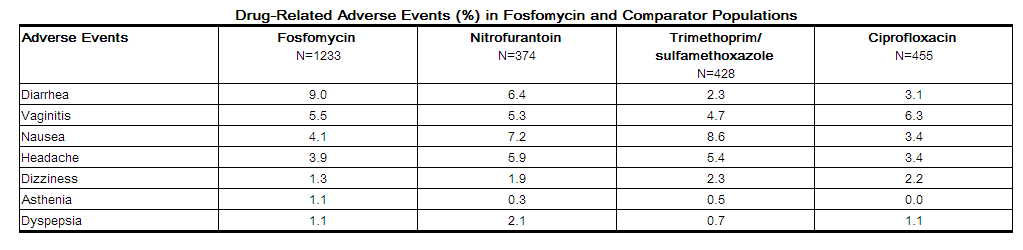
Clinical Trials:
In clinical studies, drug related adverse events which were reported in greater than 1% of the fosfomycin-treated study population are listed below:
In clinical trials, the most frequently reported adverse events occurring in > 1 % of the study population regardless of drug relationship were: diarrhea 10.4%, headache 10.3%, vaginitis 7.6%, nausea 5.2%, rhinitis 4.5%, back pain 3.0%, dysmenorrheal 2.6%, pharyngitis 2.5%, dizziness 2.3%, abdominal pain 2.2%, pain 2.2%, dyspepsia 1.8%, asthenia 1.7%, and rash 1.4%.
The following adverse events occurred in clinical trials at a rate of less than 1%, regardless of drug relationship: abnormal stools, anorexia, constipation, dry mouth, dysuria, ear disorder, fever, flatulence, flu syndrome, hematuria, infection, insomnia, lymphadenopathy, menstrual disorder, migraine, myalgia, nervousness, paresthesia, pruritus, SGPT increased, skin disorder, somnolence, and vomiting.
One patient developed unilateral optic neuritis, an event considered possibly related to MONUROL therapy.
Post-marketing Experience:
Serious adverse events from the marketing experience with MONUROL outside of the United States have been rarely reported and include: angioedema, aplastic anemia, asthma (exacerbation), cholestatic jaundice, hepatic necrosis, and toxic megacolon.
Although causality has not been established, during post marketing surveillance, the following events have occurred in patients prescribed Monurol: anaphylaxis and hearing loss.
Laboratory Changes:
Significant laboratory changes reported in U.S. clinical trials of MONUROL without regard to drug relationship include: increased eosinophil count, increased or decreased WBC count, increased bilirubin, increased SGPT, increased SGOT, increased alkaline phosphatase, decreased hematocrit, decreased hemoglobin, increased and decreased platelet count. The changes were generally transient and were not clinically significant.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf