Fondaparinux
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SPINAL/EPIDURAL HEMATOMAS
See full prescribing information for complete Boxed Warning.
Spinal/Epidural Hematomas: Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary. Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Overview
Fondaparinux is a factor Xa inhibitor that is FDA approved for the {{{indicationType}}} of prophylaxis of deep vein thrombosis (DVT) and DVT or acute pulmonary embolism (PE) when administered in conjunction with warfarin.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding complications and thrombocytopenia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Deep Vein Thrombosis Prophylaxis
- Recommended dose: 2.5 mgadministered by subcutaneous injection once daily after hemostasis has been established.
- Administer the initial dose no earlier than 6 to 8 hours after surgery.
- Administration of fondaparinux earlier than 6 hours after surgery increases the risk of major bleeding.
- The usual duration of therapy is 5 to 9 days; up to 11 days of therapy was administered in clinical trials.
- In patients undergoing hip fracture surgery, an extended prophylaxis course of up to 24 additional days is recommended.
- In patients undergoing hip fracture surgery, a total of 32 days (peri-operative and extended prophylaxis) was administered in clinical trials.
Deep Vein Thrombosis Prophylaxis Following Abdominal Surgery
- Recommended dose: 2.5 mg administered by subcutaneous injection once daily after hemostasis has been established.
- Administer the initial dose no earlier than 6 to 8 hours after surgery. Administration of fondaparinux earlier than 6 hours after surgery increases the risk of major bleeding.
- The usual duration of administration is 5 to 9 days, and up to 10 days of Fondaparinux was administered in clinical trials.
Deep Vein Thrombosis and Pulmonary Embolism Treatment
- Recommended dose: 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) by subcutaneous injection once daily
- Initiate concomitant treatment with warfarin sodium as soon as possible, usually within 72 hours.
- Continue treatment with Fondaparinux for at least 5 days and until a therapeutic oral anticoagulant effect is established (INR 2 to 3).
- The usual duration of administration of Fondaparinux is 5 to 9 days; up to 26 days of Fondaparinux injection was administered in clinical trials.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Acute ST Segment Elevation Myocardial Infarction
- Developed by: ACCF/AHA[1]
- Class of Recommendation: Class IIa
- Strength of Evidence: Category B
- Dosing Information/Recommendation
- Initial dose: 2.5 mg IV
- Maintenance dose: 2.5 mg subcutaneous daily starting the following day, for up to 8 days or until revascularization.
- Contraindicated for CrCl less than 30 mL/min.
- Fondaparinux should not be use as the sole anticoagulant for patients referred for PCI.
Acute Non-ST Segment Elevation Myocardial Infarction
- Developed by: ACCF/AHA [2]
- Class of Recommendation: Class IIb
- Strength of Evidence: Category A
- Dosing Information/Recommendation
- 2.5 mg/day SC for 8 days
Thrombosis of Superficial Vein of Lower Limb
- Developed by: American College of Chest Physicians [3]
- Class of Recommendation: Class IIb
- Strength of Evidence: Category A
- Dosing Information/Recommendation
- 2.5 mg SC for 45 days
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fondaparinux in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Fondaparinux FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fondaparinux in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fondaparinux in pediatric patients.
Contraindications
- Severe renal impairment (creatinine clearance (CrCl) < 30 mL/min).
- Active major bleeding.
- Bacterial endocarditis.
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of fondaparinux sodium.
- Body weight < 50 kg ( venous thromboembolism VTE prophylaxis only).
- History of serious hypersensitivity reaction (e.g., angioedema, anaphylactoid/anaphylactic reactions) to fondaparinux.
Warnings
|
WARNING: SPINAL/EPIDURAL HEMATOMAS
See full prescribing information for complete Boxed Warning.
Spinal/Epidural Hematomas: Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary. Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Hemorrhage
Use fondaparinux with extreme caution in conditions with increased risk of hemorrhage, such as congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, uncontrolled arterial hypertension, diabetic retinopathy, or shortly after brain, spinal, or ophthalmological surgery. Isolated cases of elevated aPTT temporally associated with bleeding events have been reported following administration of fondaparinux (with or without concomitant administration of other anticoagulants).
Do not administer agents that enhance the risk of hemorrhage with fondaparinux unless essential for the management of the underlying condition, such as vitamin K antagonists for the treatment of VTE. If co-administration is essential, closely monitor patients for signs and symptoms of bleeding.
Do not administer the initial dose of fondaparinux earlier than 6 to 8 hours after surgery. Administration earlier than 6 hours after surgery increases risk of major bleeding.
Renal Impairment and Bleeding Risk
Fondaparinux increases the risk of bleeding in patients with impaired renal function due to reduced clearance.
The incidence of major bleeding by renal function status reported in clinical trials of patients receiving fondaparinux for VTE surgical prophylaxis is provided in Table 1. In these patient populations, the following is recommended:
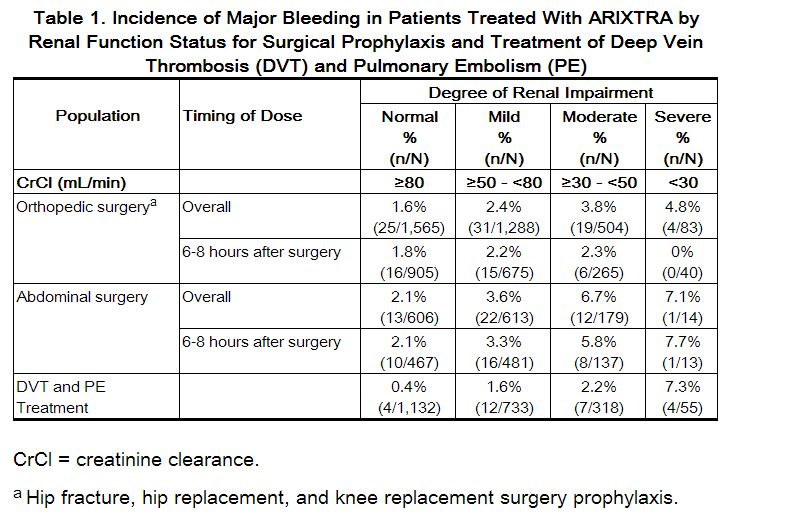
Assess renal function periodically in patients receiving fondaparinux. Discontinue the drug immediately in patients who develop severe renal impairment while on therapy. After discontinuation of fondaparinux, its anticoagulant effects may persist for 2 to 4 days in patients with normal renal function (i.e., at least 3 to 5 half-lives). The anticoagulant effects of fondaparinux may persist even longer in patients with renal impairment.
Bleeding Risk When Body Weight < 50 Kg
Fndaparinux increases the risk for bleeding in patients who weigh less than 50 kg, compared to patients with higher weights.
In patients who weigh less than 50 kg
- Do not administer fondaparinux as prophylactic therapy for patients undergoing hip fracture, hip replacement, or knee replacement surgery and abdominal surgery.
- Use fondaparinux with caution in the treatment of PE and DVT.
During randomized clinical trials of VTE prophylaxis in the peri-operative period following hip fracture, hip replacement, or knee replacement surgery and abdominal surgery, major bleeding occurred at a higher rate among patients with a body weight <50 kg compared to those with a body weight >50 kg (5.4% versus 2.1% in patients undergoing hip fracture, hip replacement, or knee replacement surgery; 5.3% versus 3.3% in patients undergoing abdominal surgery).
Thrombocytopenia
Thrombocytopenia can occur with the administration of fondaparinux. Thrombocytopenia of any degree should be monitored closely. Discontinue fondaparinux if the platelet count falls below 100,000/mm3. Moderate thrombocytopenia(platelet counts between 100,000/mm3 and 50,000/mm3) occurred at a rate of 3.0% in patients given fondaparinux 2.5 mg in the peri-operative hip fracture, hip replacement, or knee replacement surgery and abdominal surgery clinical trials. Severe thrombocytopenia (platelet counts less than 50,000/mm3) occurred at a rate of 0.2% in patients given fondaparinux 2.5 mg in these clinical trials. During extended prophylaxis, no cases of moderate or severe thrombocytopenia were reported.
Moderate thrombocytopenia occurred at a rate of 0.5% in patients given the fondaparinux treatment regimen in the DVT and PE treatment clinical trials. Severe thrombocytopenia occurred at a rate of 0.04% in patients given the fondaparinux treatment regimen in the DVT and PE treatment clinical trials.
Isolated occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported with the use of fondaparinux in postmarketing experience.
Neuraxial Anesthesia and Post-operative Indwelling Epidural Catheter Use
Spinal hematomas or epidural hematomas, which may result in long-term or permanent paralysis, can occur with the use of anticoagulants and neuraxial (spinal/epidural) anesthesia or spinal puncture. The risk of these events may be higher with post-operative use of indwelling epidural catheters or concomitant use of other drugs affecting hemostasis such as NSAIDs. In the postmarketing experience, epidural or spinal hematoma has been reported in association with the use of fondaparinux by subcutaneous (SC) injection. Monitor patients undergoing these procedures for signs and symptoms of neurologic impairment. Consider the potential risks and benefits before neuraxial intervention in patients anticoagulated or who may be anticoagulated for thromboprophylaxis.
Adverse Reactions
Clinical Trials Experience
The most serious adverse reactions reported with fondaparinux are bleeding complications and thrombocytopenia . Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reaction information below is based on data from 8,877 patients exposed to fondaparinux in controlled trials of hip fracture, hip replacement, major knee, or abdominal surgeries, and DVT and PE treatment. These trials consisted of the following:
- 2 peri-operative dose-response trials (n = 989)
- 4 active-controlled peri-operative VTE prophylaxis trials with enoxaparin sodium (n = 3,616), an extended VTE prophylaxis trial (n = 327), and an active-controlled trial with dalteparin sodium (n = 1,425)
- a dose-response trial (n = 111) and an active-controlled trial with enoxaparin sodium in DVT treatment (n = 1,091)
- an active-controlled trial with heparin in PE treatment (n = 1,092)
Hemorrhage
During administration of fondaparinux, the most common adverse reactions were bleeding complications.
Hip Fracture, Hip Replacement, and Knee Replacement Surgery
The rates of major bleeding events reported during the hip fracture, hip replacement, or knee replacement surgery clinical trials with fondaparinux 2.5 mg are provided in Table 2.
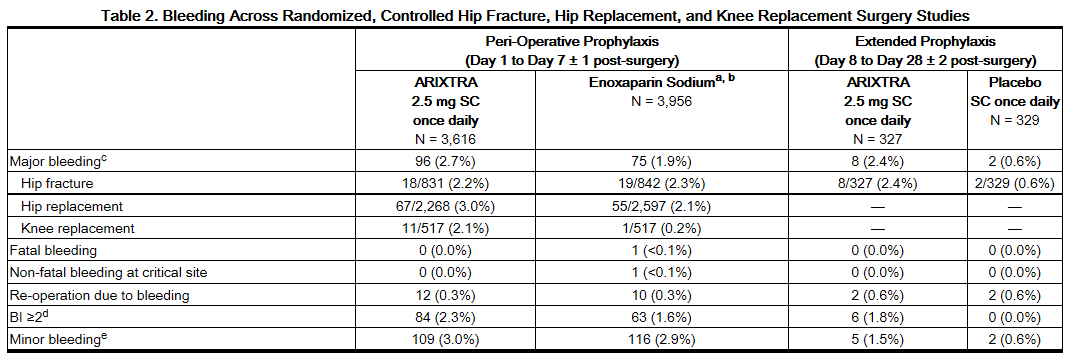
b Not approved for use in patients undergoing hip fracture surgery.
c Major bleeding was defined as clinically overt bleeding that was (1) fatal, (2) bleeding at critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), (3) associated with re-operation at operative site, or (4) with a bleeding index (BI) ≥2.
d BI ≥2: Overt bleeding associated only with a bleeding index (BI) ≥2 calculated as [number of whole blood or packed red blood cell units transfused + [(pre-bleeding) – (post-bleeding)] hemoglobin (g/dL) values].
e Minor bleeding was defined as clinically overt bleeding that was not major.
A separate analysis of major bleeding across all randomized, controlled, peri-operative, prophylaxis clinical studies of hip fracture, hip replacement, or knee replacement surgery according to the time of the first injection of fondaparinux after surgical closure was performed in patients who received fondaparinux only post-operatively. In this analysis, the incidences of major bleeding were as follows: <4 hours was 4.8% (5/104), 4 to 6 hours was 2.3% (28/1,196), 6 to 8 hours was 1.9% (38/1,965). In all studies, the majority (≥75%) of the major bleeding events occurred during the first 4 days after surgery.
Abdominal Surgery
In a randomized study of patients undergoing abdominal surgery, fondaparinux 2.5 mg once daily (n = 1,433) was compared with dalteparin 5,000 IU once daily (n = 1,425). Bleeding rates are shown in Table 3.
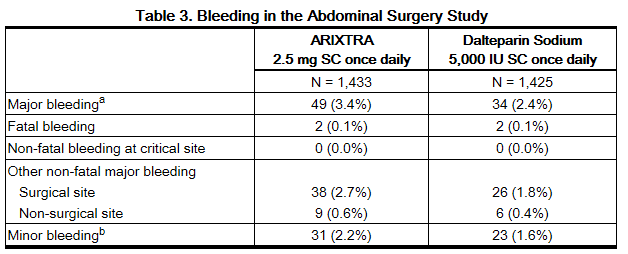
b Minor bleeding was defined as clinically overt bleeding that was not major.
The rates of major bleeding according to the time interval following the first fondaparinux injection were as follows: <6 hours was 3.4% (9/263) and 6 to 8 hours was 2.9% (32/1112).
Treatment of Deep Vein Thrombosis and Pulmonary Embolism
The rates of bleeding events reported during the DVT and PE clinical trials with the fondaparinux injection treatment regimen are provided in Table 4.
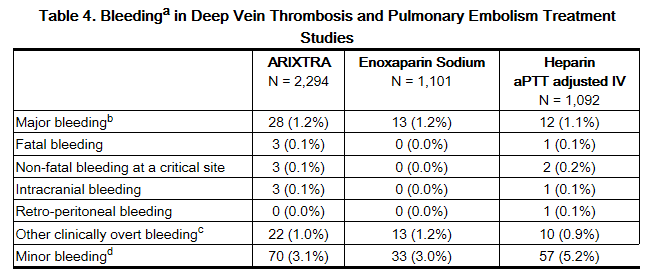
b Major bleeding was defined as clinically overt: –and/or contributing to death – and/or in a critical organ including intracranial, retroperitoneal, intraocular, spinal, pericardial, or adrenal gland – and/or associated with a fall in hemoglobin level ≥2 g/dL – and/or leading to a transfusion ≥2 units of packed red blood cells or whole blood.
c Clinically overt bleeding with a 2 g/dL fall in hemoglobin and/or leading to transfusion of PRBC or whole blood ≥2 units.
d Minor bleeding was defined as clinically overt bleeding that was not major.
Local Reactions
Local irritation (injection site bleeding, rash, and pruritus) may occur following subcutaneous injection of fondaparinux.
Elevations of Serum Aminotransferases
In the peri-operative prophylaxis randomized clinical trials of 7 ± 2 days, asymptomatic increases in aspartate (AST) and alanine (ALT) aminotransferase levels greater than 3 times the upper limit of normal were reported in 1.7% and 2.6% of patients, respectively, during treatment with fondaparinux 2.5 mg once daily versus 3.2% and 3.9% of patients, respectively, during treatment with enoxaparin sodium 30 mg every 12 hours or 40 mg once daily enoxaparin sodium. These elevations are reversible and rarely associated with increases in bilirubin. In the extended prophylaxis clinical trial, no significant differences in AST and ALT levels between fondaparinux 2.5 mg and placebo-treated patients were observed.
In the DVT and PE treatment clinical trials, asymptomatic increases in AST and ALT levels greater than 3 times the upper limit of normal of the laboratory reference range were reported in 0.7% and 1.3% of patients, respectively, during treatment with fondaparinux. In comparison, these increases were reported in 4.8% and 12.3% of patients, respectively, in the DVT treatment trial during treatment with enoxaparin sodium 1 mg/kg every 12 hours and in 2.9% and 8.7% of patients, respectively, in the PE treatment trial during treatment with aPTT adjusted heparin.
Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, elevations that might be caused by drugs like fondaparinux should be interpreted with caution.
Other Adverse Reactions
Other adverse reactions that occurred during treatment with fondaparinux in clinical trials with patients undergoing hip fracture, hip replacement, or knee replacement surgery are provided in Table 5.
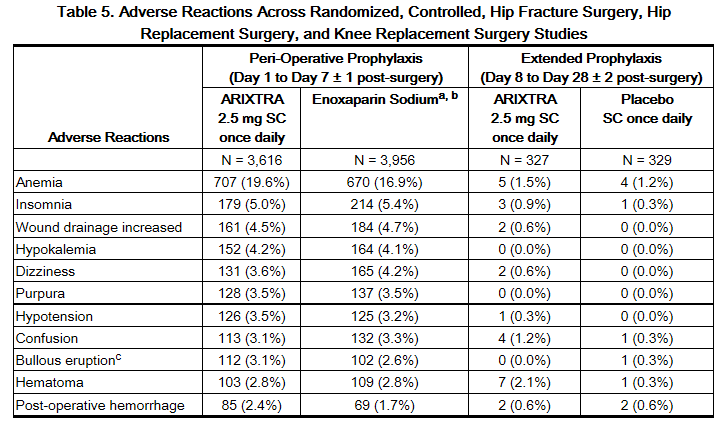
b Not approved for use in patients undergoing hip fracture surgery.
c Localized blister coded as bullous eruption.
Adverse reactions in the abdominal surgery study and in the VTE treatment trials generally occurred at lower rates than in the hip and knee surgery trials described above. The most common adverse reaction in the abdominal surgery trial was post-operative wound infection (4.9%), and the most common adverse reaction in the VTE treatment trials was epistaxis(1.3%).
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fondaparinux. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Isolated occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported in the postmarketing experience and isolated cases of elevated aPTT temporally associated with bleeding events have been reported following administration of fondaparinux (with or without concomitant administration of other anticoagulants).
Serious allergic reactions, including angioedema, anaphylactoid/anaphylactic reactions have been reported with the use of fondaparinux.
Drug Interactions
In clinical studies performed with fondaparinux, the concomitant use of oral anticoagulants (warfarin), platelet inhibitors (acetylsalicylic acid), NSAIDs(piroxicam), and digoxin did not significantly affect the pharmacokinetics/pharmacodynamics of fondaparinux sodium. In addition, fondaparinux neither influenced the pharmacodynamics of warfarin, acetylsalicylic acid, piroxicam, and digoxin, nor the pharmacokinetics of digoxin at steady state.
Agents that may enhance the risk of hemorrhage should be discontinued prior to initiation of therapy with fondaparinux unless these agents are essential. If co-administration is necessary, monitor patients closely for hemorrhage.
In an in vitro study in human liver microsomes, inhibition of CYP2A6 hydroxylation of coumarin by fondaparinux (200 micromolar i.e., 350 mg/L) was 17 to 28%. Inhibition of the other isozymes evaluated (CYPs 1A2, 2C9, 2C19, 2D6, 3A4, and 3E1) was 0 to 16%. Since fondaparinux does not markedly inhibit CYP450s (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4) in vitro, fondaparinux sodium is not expected to significantly interact with other drugs in vivo by inhibition of metabolism mediated by these isozymes.
Since fondaparinux sodium does not bind significantly to plasma proteins other than ATIII, no drug interactions by protein-binding displacement are expected.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in pregnant rats at subcutaneous doses up to 10 mg/kg/day (about 32 times the recommended human dose based on body surface area) and pregnant rabbits at subcutaneous doses up to 10 mg/kg/day (about 65 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to fondaparinux sodium. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, fondaparinux should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fondaparinux in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fondaparinux during labor and delivery.
Nursing Mothers
Fondaparinux sodium was found to be excreted in the milk of lactating rats. However, it is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when fondaparinux is administered to a nursing mother.
Pediatric Use
The pharmacokinetics of fondaparinux have not been investigated in pediatric patients.
Geriatic Use
In clinical trials the efficacy of fondaparinux in the elderly (65 years or older) was similar to that seen in patients younger than 65 years; however, serious adverse events increased with age. Exercise caution when using fondaparinux in elderly patients, paying particular attention to dosing directions and concomitant medications (especially anti-platelet medication).
Fondaparinux sodium is substantially excreted by the kidney, and the risk of adverse reactions to fondaparinux may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, assess renal function prior to fondaparinux administration.
In the peri-operative hip fracture, hip replacement, or knee replacement surgery clinical trials with patients receiving fondaparinux 2.5 mg, serious adverse events increased with age for patients receiving fondaparinux. The incidence of major bleeding in clinical trials of fondaparinux by age is provided in Table 6.
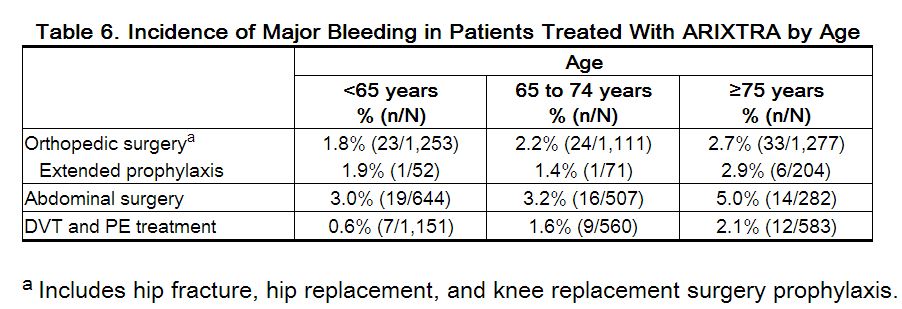
Fondaparinux elimination is prolonged in patients older than 75 years. In studies evaluating fondaparinux sodium 2.5 mg prophylaxis in hip fracture surgery or elective hip surgery, the total clearance of fondaparinux was approximately 25% lower in patients older than 75 years as compared to patients younger than 65 years. A similar relationship between fondaparinux clearance and age was observed in DVT treatment patients.
Gender
There is no FDA guidance on the use of Fondaparinux with respect to specific gender populations.
Race
Pharmacokinetic differences due to race have not been studied prospectively. However, studies performed in Asian (Japanese) healthy subjects did not reveal a different pharmacokinetic profile compared to Caucasian healthy subjects. Similarly, no plasma clearance differences were observed between black and Caucasian patients undergoing orthopedic surgery.
Renal Impairment
Fondaparinux elimination is prolonged in patients with renal impairment since the major route of elimination is urinary excretion of unchanged drug. In patients undergoing prophylaxis following elective hip surgery or hip fracture surgery, the total clearance of fondaparinux is approximately 25% lower in patients with mild renal impairment (CrCl 50 to 80 mL/min), approximately 40% lower in patients with moderate renal impairment (CrCl 30 to 50 mL/min), and approximately 55% lower in patients with severe renal impairment (<30 mL/min) compared to patients with normal renal function. A similar relationship between fondaparinux clearance and extent of renal impairment was observed in DVT treatment patients.
Hepatic Impairment
No dose adjustment is recommended in patients with mild to moderate hepatic impairment, based upon single-dose pharmacokinetic data. Pharmacokinetic data are not available for patients with severe hepatic impairment. Patients with hepatic impairment may be particularly vulnerable to bleeding during Fondaparinux therapy. Observe these patients closely for signs and symptoms of bleeding. Following a single, subcutaneous dose of 7.5 mg of fondaparinux in patients with moderate hepatic impairment (Child-Pugh Category B), Cmax and AUC were decreased by 22% and 39%, respectively, compared to subjects with normal liver function. The changes from baseline in pharmacodynamic parameters, such as aPTT, PT/INR, and antithrombin III, were similar in normal subjects and in patients with moderate hepatic impairment. Based on these data, no dosage adjustment is recommended in these patients. However, a higher incidence of hemorrhage was observed in subjects with moderate hepatic impairment than in normal subjects. The pharmacokinetics of fondaparinux have not been studied in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fondaparinux in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fondaparinux in patients who are immunocompromised.
Weight < 50kg
Total clearance of fondaparinux sodium is decreased by approximately 30% in patients weighing less than 50 kg
Administration and Monitoring
Administration
- Fondaparinux injection is provided in a single-dose, prefilled syringe affixed with an automatic needle protection system.
- Fondaparinux is administered by subcutaneous injection. It must not be administered by intramuscular injection.
- Fondaparinux is intended for use under a physician’s guidance.
- Patients may self-inject only if their physician determines that it is appropriate and the patients are trained in subcutaneous injection techniques.
- Prior to administration, visually inspect fondaparinux to ensure the solution is clear and free of particulate matter.
- To avoid the loss of drug when using the prefilled syringe, do not expel the air bubble from the syringe before the injection.
- Administration should be made in the fatty tissue, alternating injection sites (e.g., between the left and right anterolateral or the left and right posterolateral abdominal wall).
Monitoring
Routine coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) are relatively insensitive measures of the activity of fondaparinux and international standards of heparin or LMWH are not calibrators to measure anti-Factor Xa activity of fondaparinux. If unexpected changes in coagulation parameters or major bleeding occur during therapy with fondaparinux, discontinue fondaparinux. In postmarketing experience, isolated occurrences of aPTT elevations have been reported following administration of fondaparinux.
Periodic routine complete blood counts (including platelet count), serum creatinine level, and stool occult blood test are recommended during the course of treatment with fondaparinux.
The anti-factor Xa activity of fondaparinux sodium can be measured by anti-Xa assay using the appropriate calibrator (fondaparinux). The activity of fondaparinux sodium is expressed in milligrams (mg) of the fondaparinux and cannot be compared with activities of heparin or low molecular weight heparins.
IV Compatibility
There is limited information regarding the compatibility of Fondaparinux and IV administrations.
Overdosage
There is no known antidote for fondaparinux. Overdose of fondaparinux may lead to hemorrhagic complications. Discontinue treatment and initiate appropriate therapy if bleeding complications associated with overdosage occur.
Data obtained in patients undergoing chronic intermittent hemodialysis suggest that clearance of fondaparinux can increase by 20% during
Pharmacology
| |
Fondaparinux
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 1726.77 g/mol |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 94% |
| Metabolism | renally excreted unchanged |
| Half life | 17-21 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
? |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | subcutaneous |
Mechanism of Action
The antithrombotic activity of fondaparinux sodium is the result of antithrombin III (ATIII)-mediated selective inhibition of factor Xa. By selectively binding to ATIII, fondaparinux sodium potentiates (about 300 times) the innate neutralization of factor Xa by ATIII. Neutralization of factor Xa interrupts the blood coagulation cascade and thus inhibits thrombin formation and thrombus development.
Fondaparinux sodium does not inactivate thrombin (activated Factor II) and has no known effect on platelet function. At the recommended dose, fondaparinux sodium does not affect fibrinolytic activity or bleeding time.
Structure
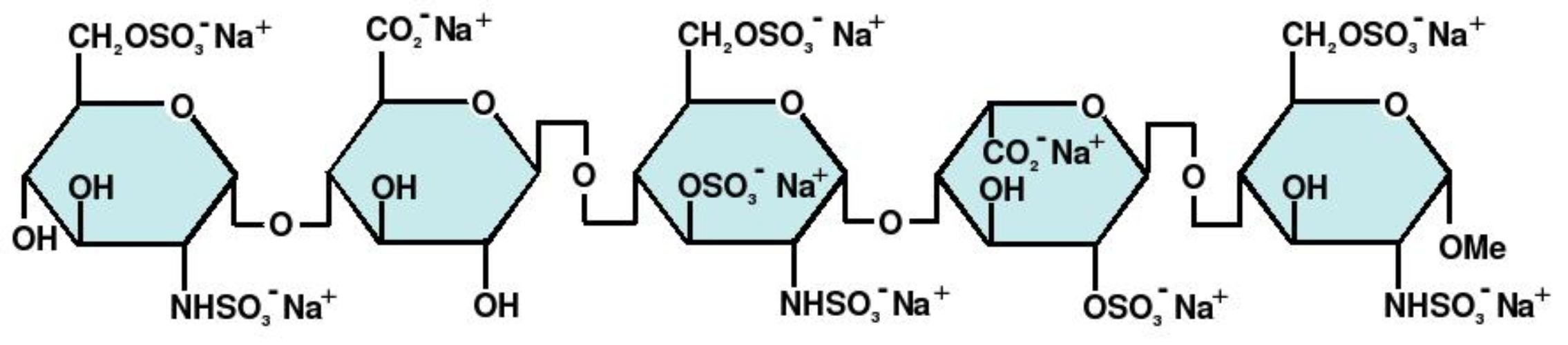
Fondaparinux sodium is a synthetic and specific inhibitor of activated factor X (Xa). Fondaparinux sodium is methyl O-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-D-glucopyranosyl-(1→4)-O-β-D-glucopyra-nuronosyl-(1→4)-O-2-deoxy-3,6-di-O-sulfo-2-(sulfoamino)-α-D-glucopyranosyl-(1→4)-O-2-O-sulfo-α-L-idopyranuronosyl-(1→4)-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-D-glucopyranoside, decasodium salt.
The molecular formula of fondaparinux sodium is C31H43N3Na10O49S8 and its molecular weight is 1728.
Pharmacodynamics
The pharmacodynamics/pharmacokinetics of fondaparinux sodium are derived from fondaparinux plasma concentrations quantified via anti-Factor Xa activity. Only fondaparinux can be used to calibrate the anti-Xa assay. (The international standards of heparin or LMWH are not appropriate for this use.) As a result, the activity of fondaparinux sodium is expressed as milligrams (mg) of the fondaparinux calibrator. The anti-Xa activity of the drug increases with increasing drug concentration, reaching maximum values in approximately three hours
Pharmacokinetics
Absorption
Fondaparinux sodium administered by subcutaneous injection is rapidly and completely absorbed (absolute bioavailability is 100%). Following a single subcutaneous dose of fondaparinux sodium 2.5 mg in young male subjects, Cmax of 0.34 mg/L is reached in approximately 2 hours. In patients undergoing treatment with fondaparinux sodium injection 2.5 mg, once daily, the peak steady-state plasma concentration is, on average, 0.39 to 0.50 mg/L and is reached approximately 3 hours post-dose. In these patients, the minimum steady-state plasma concentration is 0.14 to 0.19 mg/L. In patients with symptomatic deep vein thrombosis and pulmonary embolism undergoing treatment with fondaparinux sodium injection 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), and 10 mg (body weight >100 kg) once daily, the body–weight-adjusted doses provide similar mean steady-state peaks and minimum plasma concentrations across all body weight categories. The mean peak steady-state plasma concentration is in the range of 1.20 to 1.26 mg/L. In these patients, the mean minimum steady-state plasma concentration is in the range of 0.46 to 0.62 mg/L.
Distribution
In healthy adults, intravenously or subcutaneously administered fondaparinux sodium distributes mainly in blood and only to a minor extent in extravascular fluid as evidenced by steady state and non-steady state apparent volume of distribution of 7 to 11 L. Similar fondaparinux distribution occurs in patients undergoing elective hip surgery or hip fracture surgery. In vitro, fondaparinux sodium is highly (at least 94%) and specifically bound to antithrombin III (ATIII) and does not bind significantly to other plasma proteins (including platelet Factor 4 [PF4]) or red blood cells.
Metabolism
In vivo metabolism of fondaparinux has not been investigated since the majority of the administered dose is eliminated unchanged in urine in individuals with normal kidney function.
Elimination
In individuals with normal kidney function, fondaparinux is eliminated in urine mainly as unchanged drug. In healthy individuals up to 75 years of age, up to 77% of a single subcutaneous or intravenous fondaparinux dose is eliminated in urine as unchanged drug in 72 hours. The elimination half-life is 17 to 21 hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of fondaparinux sodium.
Fondaparinux sodium was not genotoxic in the Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, the human lymphocyte chromosome aberration test, the rat hepatocyte unscheduled DNA synthesis (UDS) test, or the rat micronucleus test.
At subcutaneous doses up to 10 mg/kg/day (about 32 times the recommended human dose based on body surface area), fondaparinux sodium was found to have no effect on fertility and reproductive performance of male and female rats.
Clinical Studies
Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
In a randomized, double-blind, clinical trial in patients undergoing hip fracture surgery, fondaparinux 2.5 mg SC once daily was compared to enoxaparin sodium 40 mg SC once daily, which is not approved for use in patients undergoing hip fracture surgery. A total of 1,711 patients were randomized and 1,673 were treated. Patients ranged in age from 17 to 101 years (mean age 77 years) with 25% men and 75% women. Patients were 99% Caucasian, 1% other races. Patients with multiple traumas affecting more than one organ system, serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. fondaparinux was initiated after surgery in 88% of patients (mean 6 hours) and enoxaparin sodium was initiated after surgery in 74% of patients (mean 18 hours). For both drugs, treatment was continued for 7 ± 2 days. The primary efficacy endpoint, venous thromboembolism (VTE), was a composite of documented deep vein thrombosis (DVT) and/or documented symptomaticpulmonary embolism (PE) reported up to Day 11. The efficacy data are provided in Table 7 and demonstrate that under the conditions of the trial fondaparinux was associated with a VTE rate of 8.3% compared with a VTE rate of 19.1% for enoxaparin sodium for a relative risk reduction of 56% (95% CI: 39%, 70%; P <0.001). Major bleeding episodes occurred in 2.2% of patients receiving fondaparinux and 2.3% of enoxaparin sodium patients [see Adverse Reactions (6.1)].
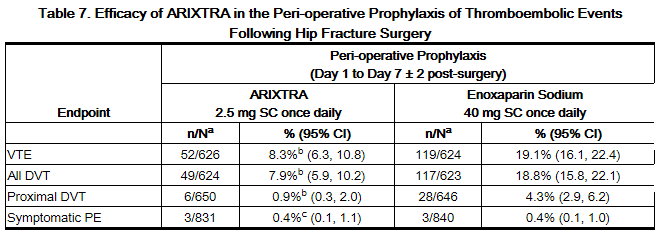
b P value versus enoxaparin sodium <0.001.
c P value versus enoxaparin sodium: NS.
Extended Prophylaxis of Thromboembolic Events Following Hip Fracture Surgery
In a noncomparative, unblinded manner, 737 patients undergoing hip fracture surgery were initially treated during the peri-operative period with fondaparinux 2.5 mg once daily for 7 ± 1 days. Eighty-one (81) of the 737 patients were not eligible for randomization into the 3-week double-blind period. Three hundred twenty-six (326) patients and 330 patients were randomized to receive fondaparinux 2.5 mg once daily or placebo, respectively, in or out of the hospital for 21 ± 2 days. Patients ranged in age from 23 to 96 years (mean age 75 years) and were 29% men and 71% women. Patients were 99% Caucasian and 1% other races. Patients with multiple traumas affecting more than one organ system or serum creatinine level more than 2 mg/dL (180 micromol/L) were excluded from the trial. The primary efficacy endpoint, venous thromboembolism (VTE), was a composite of documented deep vein thrombosis (DVT) and/or documented symptomatic pulmonary embolism (PE) reported for up to 24 days following randomization. The efficacy data are provided in Table 8 and demonstrate that extended prophylaxis with fondaparinux was associated with a VTE rate of 1.4% compared with a VTE rate of 35.0% for placebo for a relative risk reduction of 95.9% (95% CI = [98.7; 87.1], P <0.0001). Major bleeding rates during the 3-week extended prophylaxis period for fondaparinux occurred in 2.4% of patients receiving fondaparinux and 0.6% of placebo-treated patients.
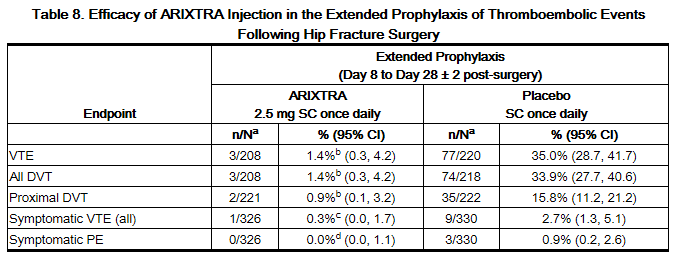
b P value versus placebo <0.001.
c P value versus placebo = 0.021.
d P value versus placebo = NS.
Prophylaxis of Thromboembolic Events Following Hip Replacement Surgery
In 2 randomized, double-blind, clinical trials in patients undergoing hip replacement surgery, fondaparinux 2.5 mg SC once daily was compared to either enoxaparin sodium 30 mg SC every 12 hours (Study 1) or to enoxaparin sodium 40 mg SC once a day (Study 2). In Study 1, a total of 2,275 patients were randomized and 2,257 were treated. Patients ranged in age from 18 to 92 years (mean age 65 years) with 48% men and 52% women. Patients were 94% Caucasian, 4% black, <1% Asian, and 2% others. In Study 2, a total of 2,309 patients were randomized and 2,273 were treated. Patients ranged in age from 24 to 97 years (mean age 65 years) with 42% men and 58% women. Patients were 99% Caucasian, and 1% other races. Patients with serum creatininelevel more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from both trials. In Study 1, fondaparinux was initiated 6 ± 2 hours (mean 6.5 hours) after surgery in 92% of patients and enoxaparin sodium was initiated 12 to 24 hours (mean 20.25 hours) after surgery in 97% of patients. In Study 2, fondaparinux was initiated 6 ± 2 hours (mean 6.25 hours) after surgery in 86% of patients and enoxaparin sodium was initiated 12 hours before surgery in 78% of patients. The first post-operative enoxaparin sodium dose was given within 12 hours after surgery in 60% of patients and 12 to 24 hours after surgery in 35% of patients with a mean of 13 hours. For both studies, both study treatments were continued for 7 ± 2 days. The efficacy data are provided in Table 9. Under the conditions of Study 1, fondaparinux was associated with a VTE rate of 6.1% compared with a VTE rate of 8.3% for enoxaparin sodium for a relative risk reduction of 26% (95% CI: -11%, 53%; P = NS). Under the conditions of Study 2, fondaparinux sodium was associated with a VTE rate of 4.1% compared with a VTE rate of 9.2% for enoxaparin sodium for a relative risk reduction of 56% (95% CI: 33%, 73%; P <0.001). For the 2 studies combined, the major bleeding episodes occurred in 3.0% of patients receiving fondaparinux and 2.1% of enoxaparin sodium patients.
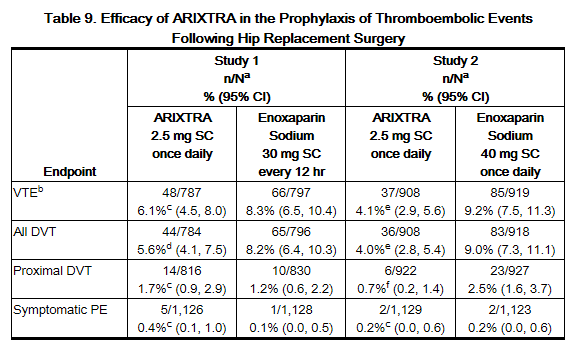
b VTE was a composite of documented DVT and/or documented symptomatic PE reported up to Day 11.
c P value versus enoxaparin sodium: NS.
d P value versus enoxaparin sodium in study 1: <0.05.
e P value versus enoxaparin sodium in study 2: <0.001.
f P value versus enoxaparin sodium in study 2: <0.01.
Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery
In a randomized, double-blind, clinical trial in patients undergoing knee replacement surgery (i.e., surgery requiring resection of the distal end of the femur or proximal end of the tibia), fondaparinux 2.5 mg SC once daily was compared to enoxaparin sodium 30 mg SC every 12 hours. A total of 1,049 patients were randomized and 1,034 were treated. Patients ranged in age from 19 to 94 years (mean age 68 years) with 41% men and 59% women. Patients were 88% Caucasian, 8% black, <1% Asian, and 3% others. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. fondaparinux was initiated 6 ± 2 hours (mean 6.25 hours) after surgery in 94% of patients, and enoxaparin sodium was initiated 12 to 24 hours (mean 21 hours) after surgery in 96% of patients. For both drugs, treatment was continued for 7 ± 2 days. The efficacy data are provided in Table 10 and demonstrate that under the conditions of the trial, fondaparinux was associated with a VTE rate of 12.5% compared with a VTE rate of 27.8% for enoxaparin sodium for a relative risk reduction of 55% (95% CI: 36%, 70%; P <0.001). Major bleeding episodes occurred in 2.1% of patients receiving fondaparinux and 0.2% of enoxaparin sodium patients.
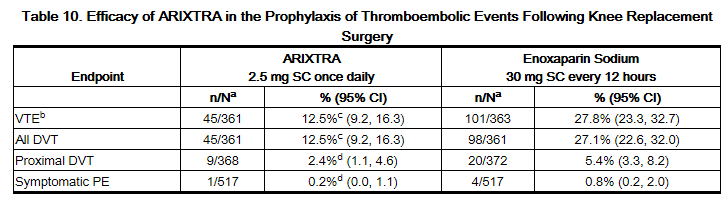
Prophylaxis of Thromboembolic Events Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
Abdominal surgery patients at risk included the following: Those undergoing surgery under general anesthesia lasting longer than 45 minutes who are older than 60 years with or without additional risk factors; and those undergoing surgery under general anesthesia lasting longer than 45 minutes who are older than 40 years with additional risk factors. Risk factors included neoplastic disease, obesity, chronic obstructive pulmonary disease, inflammatory bowel disease, history of deep vein thrombosis (DVT) or pulmonary embolism (PE), or congestive heart failure.
In a randomized, double-blind, clinical trial in patients undergoing abdominal surgery, fondaparinux 2.5 mg SC once daily started postoperatively was compared to dalteparin sodium 5,000 IU SC once daily, with one 2,500 IU SC preoperative injection and a 2,500 IU SC first postoperative injection. A total of 2,927 patients were randomized and 2,858 were treated. Patients ranged in age from 17 to 93 years (mean age 65 years) with 55% men and 45% women. Patients were 97% Caucasian, 1% black, 1% Asian, and 1% others. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. Sixty-nine percent (69%) of study patients underwent cancer-related abdominal surgery. Study treatment was continued for 7 ± 2 days. The efficacy data are provided in Table 11 and demonstrate that prophylaxis with fondaparinux was associated with a VTE rate of 4.6% compared with a VTE rate of 6.1% for dalteparin sodium (P = NS).
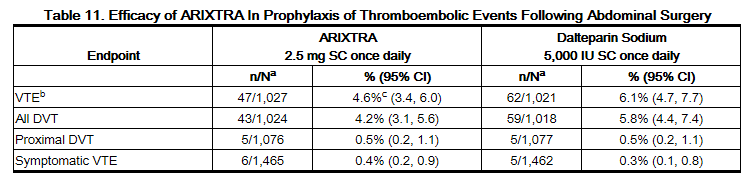
b VTE was a composite of venogram positive DVT, symptomatic DVT, non-fatal PE and/or fatal PE reported up to Day 10.
c P value versus dalteparin sodium: NS.
Treatment of Deep Vein Thrombosis
In a randomized, double-blind, clinical trial in patients with a confirmed diagnosis of acute symptomatic DVT without PE, fondaparinux 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) SC once daily (fondaparinux treatment regimen) was compared to enoxaparinsodium 1 mg/kg SC every 12 hours. Almost all patients started study treatment in hospital. Approximately 30% of patients in both groups were discharged home from the hospital while receiving study treatment. A total of 2,205 patients were randomized and 2,192 were treated. Patients ranged in age from 18 to 95 years (mean age 61 years) with 53% men and 47% women. Patients were 97% Caucasian, 2% black, and 1% other races. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. For both groups, treatment continued for at least 5 days with a treatment duration range of 7 ± 2 days, and both treatment groups received vitamin K antagonist therapy initiated within 72 hours after the first study drug administration and continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. The primary efficacy endpoint was confirmed, symptomatic, recurrent VTE reported up to Day 97. The efficacy data are provided in Table 12.
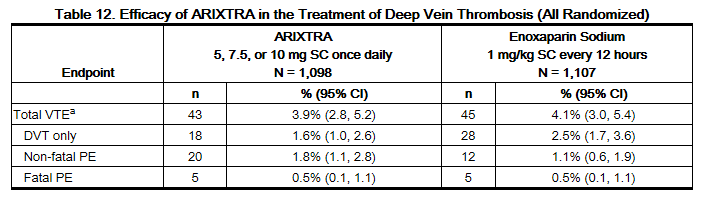
During the initial treatment period, 18 (1.6%) of patients treated with fondaparinux sodium and 10 (0.9%) of patients treated with enoxaparin sodium had a VTE endpoint (95% CI for the treatment difference [fondaparinux sodium-enoxaparin sodium] for VTE rates: -0.2%; 1.7%).
During the initial treatment period, 18 (1.6%) of patients treated with fondaparinux sodium and 10 (0.9%) of patients treated with enoxaparin sodium had a VTE endpoint (95% CI for the treatment difference [fondaparinux sodium-enoxaparin sodium] for VTE rates: -0.2%; 1.7%).
Treatment of Pulmonary Embolism
In a randomized, open-label, clinical trial in patients with a confirmed diagnosis of acute symptomatic PE, with or without DVT, fondaparinux 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) SC once daily (fondaparinux treatment regimen) was compared to heparin IV bolus (5,000 USP units) followed by a continuous IV infusion adjusted to maintain 1.5 to 2.5 times aPTT control value. Patients with a PE requiring thrombolysis or surgical thrombectomy were excluded from the trial. All patients started study treatment in hospital. Approximately 15% of patients were discharged home from the hospital while receiving fondaparinux therapy. A total of 2,213 patients were randomized and 2,184 were treated. Patients ranged in age from 18 to 97 years (mean age 62 years) with 44% men and 56% women. Patients were 94% Caucasian, 5% black, and 1% other races. Patients with serum creatinine level more than 2 mg/dL (180 micromol/L), or platelet count less than 100,000/mm3 were excluded from the trial. For both groups, treatment continued for at least 5 days with a treatment duration range 7 ± 2 days, and both treatment groups received vitamin K antagonist therapy initiated within 72 hours after the first study drug administration and continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. The primary efficacy endpoint was confirmed, symptomatic, recurrent VTE reported up to Day 97. The efficacy data are provided in Table 13.
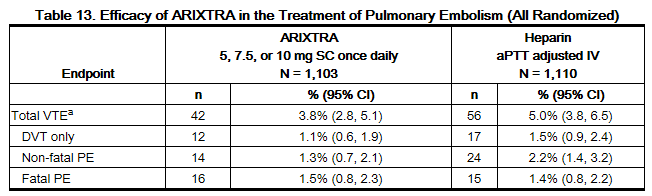
During the initial treatment period, 12 (1.1%) of patients treated with fondaparinux sodium and 19 (1.7%) of patients treated with heparin had a VTE endpoint (95% CI for the treatment difference [fondaparinux sodium-heparin] for VTE rates: -1.6%; 0.4%).
How Supplied
Fondaparinux injection is available in the following strengths and package sizes:
- 2.5 mg fondaparinux in 0.5 mL single-dose prefilled syringe, affixed with a 27-gauge x ½-inch needle and an automatic needle protection system with white plunger rod.
- 5 mg fondaparinux in 0.4 mL single-dose prefilled syringe, affixed with a 27-gauge x ½-inch needle and an automatic needle protection system with white plunger rod.
- 7.5 mg fondaparinux in 0.6 mL single-dose prefilled syringe, affixed with a 27-gauge x ½-inch needle and an automatic needle protection system with white plunger rod.
- 10 mg fondaparinux in 0.8 mL single-dose prefilled syringe, affixed with a 27-gauge x ½-inch needle and an automatic needle protection system with white plunger rod.
Storage
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F).
Images
Drug Images
{{#ask: Page Name::Fondaparinux |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fondaparinux |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
If the patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDS, platelet inhibitors, or other anticoagulants, they should be informed to watch for signs and symptoms of spinal or epidural hematomas, such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur, the patients should contact his or her physician immediately.
The use of aspirin and other NSAIDS may enhance the risk of hemorrhage. Their use should be discontinued prior to fondaparinux therapy whenever possible; if co-administration is essential, the patient’s clinical and laboratory status should be closely monitored. [See Drug Interactions (7).]
If patients must self-administer fondaparinux (e.g., if fondaparinux is used at home), they should be advised of the following:
- Fondaparinux should be given by subcutaneous injection. Patients must be instructed in the proper technique for administration.
- As with all anticoagulants, the most important risk with fondaparinux administration is bleeding. Patients should be counseled on signs and symptoms of possible bleeding.
- It may take them longer than usual to stop bleeding.
- They may bruise and/or bleed more easily when they are treated with fondaparinux.
- They should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician.
- To tell their physicians and dentists they are taking fondaparinuxand/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken.
- To tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs.
Keep out of the reach of children.
Precautions with Alcohol
Alcohol-Fondaparinux interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Arixtra
Look-Alike Drug Names
- Arixtra - Arista AH
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Patrick T. O'Gara, Frederick G. Kushner, Deborah D. Ascheim, Donald E. Jr Casey, Mina K. Chung, James A. de Lemos, Steven M. Ettinger, James C. Fang, Francis M. Fesmire, Barry A. Franklin, Christopher B. Granger, Harlan M. Krumholz, Jane A. Linderbaum, David A. Morrow, L. Kristin Newby, Joseph P. Ornato, Narith Ou, Martha J. Radford, Jacqueline E. Tamis-Holland, Carl L. Tommaso, Cynthia M. Tracy, Y. Joseph Woo, David X. Zhao, Jeffrey L. Anderson, Alice K. Jacobs, Jonathan L. Halperin, Nancy M. Albert, Ralph G. Brindis, Mark A. Creager, David DeMets, Robert A. Guyton, Judith S. Hochman, Richard J. Kovacs, Frederick G. Kushner, E. Magnus Ohman, William G. Stevenson & Clyde W. Yancy (2013). "2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation. 127 (4): e362–e425. doi:10.1161/CIR.0b013e3182742cf6. PMID 23247304. Unknown parameter
|month=ignored (help) - ↑ Jeffrey L. Anderson, Cynthia D. Adams, Elliott M. Antman, Charles R. Bridges, Robert M. Califf, Donald E. Jr Casey, William E. 2nd Chavey, Francis M. Fesmire, Judith S. Hochman, Thomas N. Levin, A. Michael Lincoff, Eric D. Peterson, Pierre Theroux, Nanette K. Wenger, R. Scott Wright, Hani Jneid, Steven M. Ettinger, Theodore G. Ganiats, A. Michael Lincoff, George J. Philippides & James Patrick Zidar (2013). "2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation. 127 (23): e663–e828. doi:10.1161/CIR.0b013e31828478ac. PMID 23630129. Unknown parameter
|month=ignored (help) - ↑ Clive Kearon, Elie A. Akl, Anthony J. Comerota, Paolo Prandoni, Henri Bounameaux, Samuel Z. Goldhaber, Michael E. Nelson, Philip S. Wells, Michael K. Gould, Francesco Dentali, Mark Crowther & Susan R. Kahn (2012). "Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e419S–e494S. doi:10.1378/chest.11-2301. PMID 22315268. Unknown parameter
|month=ignored (help)
{{#subobject:
|Label Page=Fondaparinux |Label Name=Fondaparinux 2.5.jpg
}}
{{#subobject:
|Label Page=Fondaparinux |Label Name=Fondaparinux 5.jpg
}}
{{#subobject:
|Label Page=Fondaparinux |Label Name=Fondaparinux 7.5.jpg
}}
{{#subobject:
|Label Page=Fondaparinux |Label Name=Fondaparinux 10.jpg
}}