Ezetimibe/Simvastatin clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Studies
Primary hyperlipidemia
VYTORIN
VYTORIN reduces total-C, LDL-C, Apo B, TG, and non-HDL-C, and increases HDL-C in patients with hyperlipidemia. Maximal to near maximal response is generally achieved within 2 weeks and maintained during chronic therapy.
VYTORIN is effective in men and women with hyperlipidemia. Experience in non-Caucasians is limited and does not permit a precise estimate of the magnitude of the effects of VYTORIN.
Five multicenter, double-blind studies conducted with either VYTORIN or coadministered ezetimibe and simvastatin equivalent to VYTORIN in patients with primary hyperlipidemia are reported: two were comparisons with simvastatin, two were comparisons with atorvastatin, and one was a comparison with rosuvastatin.
In a multicenter, double-blind, placebo-controlled, 12-week trial, 1528 hyperlipidemic patients were randomized to one of ten treatment groups: placebo, ezetimibe (10 mg), simvastatin (10 mg, 20 mg, 40 mg, or 80 mg), or VYTORIN (10/10, 10/20, 10/40, or 10/80).
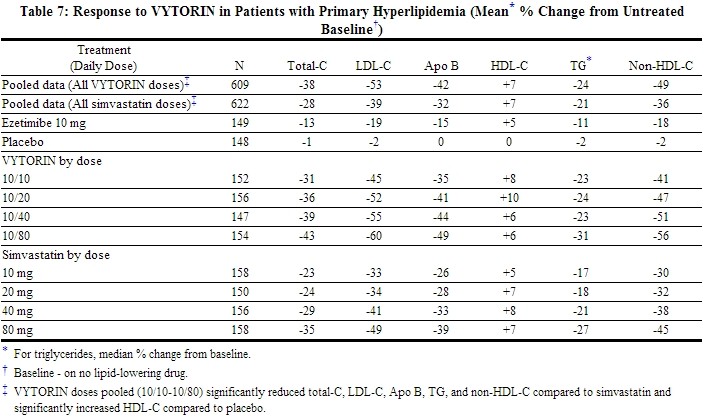
When patients receiving VYTORIN were compared to those receiving all doses of simvastatin, VYTORIN significantly lowered total-C, LDL-C, Apo B, TG, and non-HDL-C. The effects of VYTORIN on HDL-C were similar to the effects seen with simvastatin. Further analysis showed VYTORIN significantly increased HDL-C compared with placebo. (See Table 7.) The lipid response to VYTORIN was similar in patients with TG levels greater than or less than 200 mg/dL.
 |
In a multicenter, double-blind, controlled, 23-week study, 710 patients with known CHD or CHD risk equivalents, as defined by the NCEP ATP III guidelines, and an LDL-C ≥130 mg/dL were randomized to one of four treatment groups: coadministered ezetimibe and simvastatin equivalent to VYTORIN (10/10, 10/20, and 10/40) or simvastatin 20 mg. Patients not reaching an LDL-C <100 mg/dL had their simvastatin dose titrated at 6-week intervals to a maximal dose of 80 mg.
At Week 5, the LDL-C reductions with VYTORIN 10/10, 10/20, or 10/40 were significantly larger than with simvastatin 20 mg (see Table 8).
 |
In a multicenter, double-blind, 6-week study, 1902 patients with primary hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were randomized to one of eight treatment groups: VYTORIN (10/10, 10/20, 10/40, or 10/80) or atorvastatin (10 mg, 20 mg, 40 mg, or 80 mg).
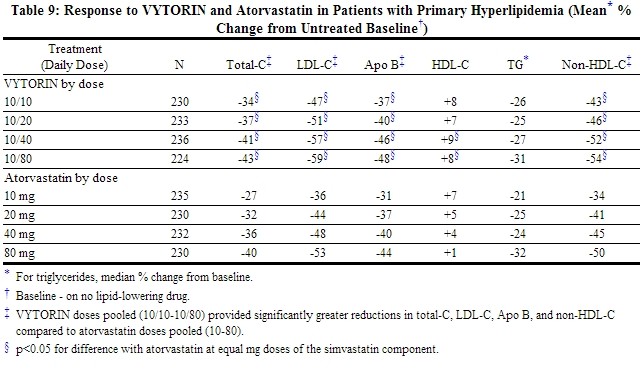
Across the dosage range, when patients receiving VYTORIN were compared to those receiving milligram-equivalent statin doses of atorvastatin, VYTORIN lowered total-C, LDL-C, Apo B, and non-HDL-C significantly more than atorvastatin. Only the 10/40 mg and 10/80 mg VYTORIN doses increased HDL-C significantly more than the corresponding milligram-equivalent statin dose of atorvastatin. The effects of VYTORIN on TG were similar to the effects seen with atorvastatin. (See Table 9.)
 |
In a multicenter, double-blind, 24-week, forced-titration study, 788 patients with primary hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were randomized to receive coadministered ezetimibe and simvastatin equivalent to VYTORIN (10/10 and 10/20) or atorvastatin 10 mg. For all three treatment groups, the dose of the statin was titrated at 6-week intervals to 80 mg. At each pre-specified dose comparison, VYTORIN lowered LDL-C to a greater degree than atorvastatin (see Table 10).
 |
In a multicenter, double-blind, 6-week study, 2959 patients with primary hyperlipidemia, who had not met their NCEP ATP III target LDL-C goal, were randomized to one of six treatment groups: VYTORIN (10/20, 10/40, or 10/80) or rosuvastatin (10 mg, 20 mg, or 40 mg).
The effects of VYTORIN and rosuvastatin on total-C, LDL-C, Apo B, TG, non-HDL-C and HDL-C are shown in Table 11.
 |
In a multicenter, double-blind, 24-week trial, 214 patients with type 2 diabetes mellitus treated with thiazolidinediones (rosiglitazone or pioglitazone) for a minimum of 3 months and simvastatin 20 mg for a minimum of 6 weeks were randomized to receive either simvastatin 40 mg or the coadministered active ingredients equivalent to VYTORIN 10/20. The median LDL-C and HbA1c levels at baseline were 89 mg/dL and 7.1%, respectively.
VYTORIN 10/20 was significantly more effective than doubling the dose of simvastatin to 40 mg. The median percent changes from baseline for VYTORIN vs. simvastatin were: LDL-C -25% and -5%; total-C -16% and -5%; Apo B -19% and -5%; and non-HDL-C -23% and -5%. Results for HDL-C and TG between the two treatment groups were not significantly different.
Ezetimibe
In two multicenter, double-blind, placebo-controlled, 12-week studies in 1719 patients with primary hyperlipidemia, ezetimibe significantly lowered total-C (-13%), LDL-C (-19%), Apo B (-14%), and TG (-8%), and increased HDL-C (+3%) compared to placebo. Reduction in LDL-C was consistent across age, sex, and baseline LDL-C.
Simvastatin
In two large, placebo-controlled clinical trials, the Scandinavian Simvastatin Survival Study (N=4,444 patients) and the Heart Protection Study (N=20,536 patients), the effects of treatment with simvastatin were assessed in patients at high risk of coronary events because of existing coronary heart disease, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease. Simvastatin was proven to reduce: the risk of total mortality by reducing CHD deaths; the risk of non-fatal myocardial infarction and stroke; and the need for coronary and non-coronary revascularization procedures.
No incremental benefit of VYTORIN on cardiovascular morbidity and mortality over and above that demonstrated for simvastatin has been established.
Homozygous Familial Hypercholesterolemia (HoFH)
A double-blind, randomized, 12-week study was performed in patients with a clinical and/or genotypic diagnosis of HoFH. Data were analyzed from a subgroup of patients (n=14) receiving simvastatin 40 mg at baseline. Increasing the dose of simvastatin from 40 to 80 mg (n=5) produced a reduction of LDL-C of 13% from baseline on simvastatin 40 mg. Coadministered ezetimibe and simvastatin equivalent to VYTORIN (10/40 and 10/80 pooled, n=9), produced a reduction of LDL-C of 23% from baseline on simvastatin 40 mg. In those patients coadministered ezetimibe and simvastatinequivalent to VYTORIN (10/80, n=5), a reduction of LDL-C of 29% from baseline on simvastatin 40 mg was produced.
Chronic Kidney Disease (CKD)
The Study of Heart and Renal Protection (SHARP) was a multinational, randomized, placebo-controlled, double-blind trial that investigated the effect of VYTORIN on the time to a first major vascular event (MVE) among 9438 patients with moderate to severe chronic kidney disease (approximately one-third on dialysis at baseline) who did not have a history of myocardial infarction or coronary revascularization. An MVE was defined as nonfatal MI, cardiac death, stroke, or any revascularization procedure. Patients were allocated to treatment using a method that took into account the distribution of 8 important baseline characteristics of patients already enrolled and minimized the imbalance of those characteristics across the groups.
For the first year, 9438 patients were allocated 4:4:1, to VYTORIN 10/20, placebo, or simvastatin 20 mg daily, respectively. The 1-year simvastatinarm enabled the comparison of VYTORIN to simvastatin with regard to safety and effect on lipid levels. At 1 year the simvastatin-only arm was re-allocated 1:1 to VYTORIN 10/20 or placebo. A total of 9270 patients were ever allocated to VYTORIN 10/20 (n=4650) or placebo (n=4620) during the trial. The median follow-up duration was 4.9 years. Patients had a mean age of 61 years; 63% were male, 72% were Caucasian, and 23% were diabetic; and, for those not on dialysis at baseline, the median serum creatinine was 2.5 mg/dL and the median estimated glomerular filtration rate (eGFR) was 25.6 mL/min/1.73 m2, with 94% of patients having an eGFR < 45 mL/min/1.73m2. Eligibility did not depend on lipid levels. Mean LDL-C at baseline was 108 mg/dL. At 1 year, the mean LDL-C was 26% lower in the simvastatin arm and 38% lower in the VYTORIN arm relative to placebo. At the midpoint of the study (2.5 years), the mean LDL-C was 32% lower for VYTORIN relative to placebo. Patients no longer taking study medication were included in all lipid measurements.
In the primary intent-to-treat analysis, 639 (15.2%) of 4193 patients initially allocated to VYTORIN and 749 (17.9%) of 4191 patients initially allocated to placebo experienced an MVE. This corresponded to a relative risk reduction of 16% (p=0.001) (see Figure 1). Similarly, 526 (11.3%) of 4650 patients ever allocated to VYTORIN and 619 (13.4%) of 4620 patients ever allocated to placebo experienced a major atherosclerotic event (MAE; a subset of the MVE composite that excluded non-coronary cardiac deaths and hemorrhagic stroke), corresponding to a relative risk reduction of 17% (p=0.002). The trial demonstrated that treatment with VYTORIN 10/20 mg versus placebo reduced the risk for MVE and MAE in this CKD population. The study design precluded drawing conclusions regarding the independent contribution of either ezetimibe or simvastatin to the observed effect.
The treatment effect of VYTORIN on MVE was attenuated among patients on dialysis at baseline compared with those not on dialysis at baseline. Among 3023 patients on dialysis at baseline, VYTORIN reduced the risk of MVE by 6% (RR 0.94: 95% CI 0.80-1.09) compared with 22% (RR 0.78: 95% CI 0.69-0.89) among 6247 patients not on dialysis at baseline (interaction P=0.08).
 |
 |
Among patients not on dialysis at baseline, VYTORIN did not reduce the risk of progressing to end-stage renal disease compared with placebo (RR 0.97: 95% CI 0.89-1.05).[1]
References
- ↑ "VYTORIN (EZETIMIBE AND SIMVASTATIN) TABLET [MERCK SHARP & DOHME CORP.]". Retrieved 19 February 2014.