Etravirine clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Pharmacology
Mechanism of Action
Etravirine is an antiviral drug.
Pharmacodynamics
Effects on Electrocardiogram
In a randomized, double-blind, active, and placebo-controlled crossover study, 41 healthy subjects were administered INTELENCE® 200 mg twice daily, INTELENCE® 400 mg once daily, placebo, and moxifloxacin 400 mg. After 8 days of dosing, etravirine did not prolong the QT interval. The maximum mean (upper 1-sided 95% CI) baseline and placebo-adjusted QTcF were 0.6 ms (3.3 ms) for 200 mg twice daily and -1.0 ms (2.5 ms) for 400 mg once daily dosing regimens.
Pharmacokinetics in Adults
The pharmacokinetic properties of INTELENCE® were determined in healthy adult subjects and in treatment-experienced HIV-1-infected adult and pediatric subjects. The systemic exposures (AUC) to etravirine were lower in HIV-1-infected subjects than in healthy subjects.
 |
Note: The median protein binding adjusted EC50 for MT4 cells infected with HIV-1/IIIB in vitro equals 4 ng per mL.
Absorption and Bioavailability
Following oral administration, etravirine was absorbed with a Tmax of about 2.5 to 4 hours. The absolute oral bioavailability of INTELENCE® is unknown.
In healthy subjects, the absorption of etravirine is not affected by co-administration of oral ranitidine or omeprazole, drugs that increase gastric pH.
Effects of Food on Oral Absorption
The systemic exposure (AUC) to etravirine was decreased by about 50% when INTELENCE® was administered under fasting conditions, as compared to when INTELENCE® was administered following a meal. Therefore, INTELENCE® should always be taken following a meal. Within the range of meals studied, the systemic exposures to etravirine were similar. The total caloric content of the various meals evaluated ranged from 345 kilocalories (17 grams fat) to 1160 kilocalories (70 grams fat) [see Dosage and Administration (2)].
Distribution
Etravirine is about 99.9% bound to plasma proteins, primarily to albumin (99.6%) and alpha 1-acid glycoprotein (97.66% to 99.02%) in vitro. The distribution of etravirine into compartments other than plasma (e.g., cerebrospinal fluid, genital tract secretions) has not been evaluated in humans.
Metabolism
In vitro experiments with human liver microsomes (HLMs) indicate that etravirine primarily undergoes metabolism by CYP3A, CYP2C9, and CYP2C19 enzymes. The major metabolites, formed by methyl hydroxylation of the dimethylbenzonitrile moiety, were at least 90% less active than etravirine against wild-type HIV in cell culture.
Elimination
After single dose oral administration of 800 mg 14C-etravirine, 93.7% and 1.2% of the administered dose of 14C-etravirine was recovered in the feces and urine, respectively. Unchanged etravirine accounted for 81.2% to 86.4% of the administered dose in feces. Unchanged etravirine was not detected in urine. The mean (± standard deviation) terminal elimination half-life of etravirine was about 41 (± 20) hours.
Special Populations
Hepatic Impairment
Etravirine is primarily metabolized by the liver. The steady state pharmacokinetic parameters of etravirine were similar after multiple dose administration of INTELENCE® to subjects with normal hepatic function (16 subjects), mild hepatic impairment (Child-Pugh Class A, 8 subjects), and moderate hepatic impairment (Child-Pugh Class B, 8 subjects). The effect of severe hepatic impairment on the pharmacokinetics of etravirine has not been evaluated.
Hepatitis B and/or Hepatitis C Virus Co-infection
Population pharmacokinetic analysis of the TMC125-C206 and TMC125-C216 trials showed reduced clearance for etravirine in HIV-1-infected subjects with hepatitis B and/or C virus co-infection. Based upon the safety profile of INTELENCE® [see Adverse Reactions (6)], no dose adjustment is necessary in patients co-infected with hepatitis B and/or C virus.
Renal Impairment
The pharmacokinetics of etravirine have not been studied in patients with renal impairment. The results from a mass balance study with 14C-etravirine showed that less than 1.2% of the administered dose of etravirine is excreted in the urine as metabolites. No unchanged drug was detected in the urine. As etravirine is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Gender
No significant pharmacokinetic differences have been observed between males and females.
Race
Population pharmacokinetic analysis of etravirine in HIV-infected subjects did not show an effect of race on exposure to etravirine.
Geriatric Patients
Population pharmacokinetic analysis in HIV-infected subjects showed that etravirine pharmacokinetics are not considerably different within the age range (18 to 77 years) evaluated [see Use in Specific Populations (8.5)].
Pediatric Patients
The pharmacokinetics of etravirine in 101 treatment-experienced HIV-1-infected pediatric subjects, 6 years to less than 18 years of age and weighing at least 16 kg showed that the administered weight-based dosages (approximately 5.2 mg per kg twice daily up to the adult recommended doses) resulted in etravirine exposure comparable to that in adults receiving INTELENCE® 200 mg twice daily when administered at a dose corresponding to 5.2 mg per kg twice daily. The population pharmacokinetic estimates for etravirine AUC12h and C0h are summarized in the table below.
 |
The pharmacokinetics of etravirine in pediatric subjects less than 6 years of age have not been established.
Etravirine is a substrate of CYP3A, CYP2C9, and CYP2C19. Therefore, co-administration of INTELENCE® with drugs that induce or inhibit CYP3A, CYP2C9, and CYP2C19 may alter the therapeutic effect or adverse reaction profile of INTELENCE®.
Etravirine is an inducer of CYP3A and inhibitor of CYP2C9, CYP2C19 and P-glycoprotein. Therefore, co-administration of drugs that are substrates of CYP3A, CYP2C9 and CYP2C19 or are transported by P-glycoprotein with INTELENCE® may alter the therapeutic effect or adverse reaction profile of the co-administered drug(s).
Drug interaction studies were performed with INTELENCE® and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of other drugs on the AUC, Cmax, and Cmin values of etravirine are summarized in Table 5 (effect of other drugs on INTELENCE®). The effect of co-administration of INTELENCE® on the AUC, Cmax, and Cmin values of other drugs are summarized in Table 6 (effect of INTELENCE® on other drugs).[1]
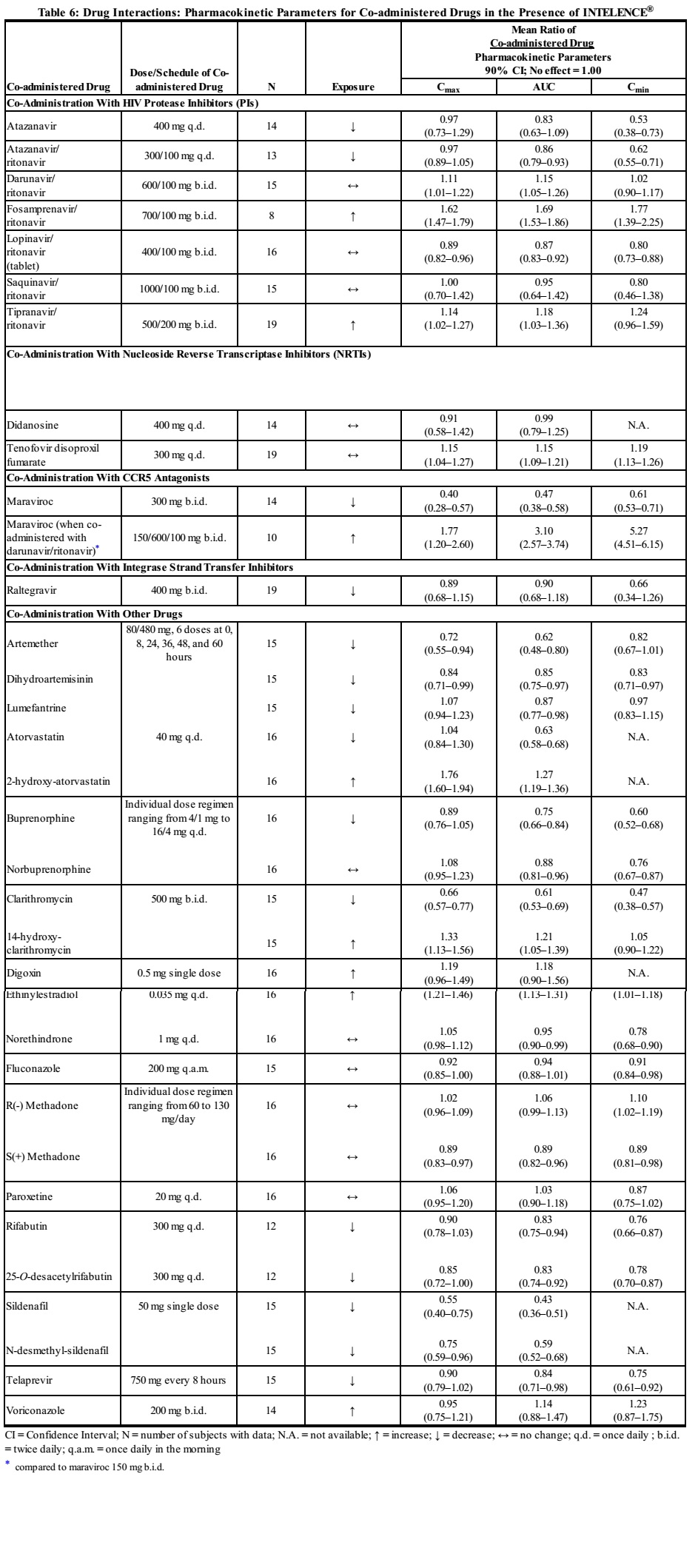 |
References
Adapted from the FDA Package Insert.