Epoetin alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
See full prescribing information for complete Boxed Warning.
|
Overview
Epoetin alfa is an erythropoiesis-stimulating agent (ESA) that is FDA approved for the {{{indicationType}}} of anemia due to chronic kidney disease, anemia due to zidovudine in hiv-infected patients, anemia due to chemotherapy in patients with cancer, reduction of allogeneic red blood cell transfusions in patients undergoing elective, noncardiac, nonvascular surgery. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, injection site irritation, injection site pain, pruritus, rash, nausea, vomiting, arthralgia, myalgia, dizziness, headache, insomnia, cough, upper respiratory infection, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Evaluate the iron status in all patients before and during treatment and maintain iron repletion. Correct or exclude other causes of anemia (e.g., vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding, etc.) before initiating Epogen
Chronic Kidney Disease
- Epogen is indicated for the treatment of anemia due to chronic kidney disease (CKD), including patients on dialysis and not on dialysis to decrease the need for red blood cell (RBC) transfusion.
- In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL. No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. Individualize dosing and use the lowest dose of Epogen sufficient to reduce the need for RBC transfusions. Physicians and patients should weigh the possible benefits of decreasing transfusions against the increased risks of death and other serious cardiovascular adverse events.
For all patients with CKD
- When initiating or adjusting therapy, monitor hemoglobin levels at least weekly until stable, then monitor at least monthly. When adjusting therapy consider hemoglobin rate of rise, rate of decline, ESA responsiveness and hemoglobin variability. A single hemoglobin excursion may not require a dosing change.
- Do not increase the dose more frequently than once every 4 weeks. Decreases in dose can occur more frequently. Avoid frequent dose adjustments.
- If the hemoglobin rises rapidly (e.g., more than 1 g/dL in any 2-week period), reduce the dose of Epogen by 25% or more as needed to reduce rapid responses.
- For patients who do not respond adequately, if the hemoglobin has not increased by more than 1 g/dL after 4 weeks of therapy, increase the dose by 25%.
- For patients who do not respond adequately over a 12-week escalation period, increasing the Epogen dose further is unlikely to improve response and may increase risks. Use the lowest dose that will maintain a hemoglobin level sufficient to reduce the need for RBC transfusions. Evaluate other causes of anemia. Discontinue Epogen if responsiveness does not improve.
For patients with CKD on dialysis
- Initiate Epogen treatment when the hemoglobin level is less than 10 g/dL.
- If the hemoglobin level approaches or exceeds 11 g/dL, reduce or interrupt the dose of Epogen.
- The recommended starting dose for adult patients is 50 to 100 Units/kg 3 times weekly intravenously or subcutaneously. For pediatric patients, a starting dose of 50 Units/kg 3 times weekly intravenously or subcutaneously is recommended. The intravenous route is recommended for patients on hemodialysis.
For patients with CKD not on dialysis
- Consider initiating Epogen treatment only when the hemoglobin level is less than 10 g/dL and the following considerations apply:
- The rate of hemoglobin decline indicates the likelihood of requiring a RBC transfusion and,
- Reducing the risk of alloimmunization and/or other RBC transfusion-related risks is a goal
- If the hemoglobin level exceeds 10 g/dL, reduce or interrupt the dose of Epogen, and use the lowest dose of Epogen sufficient to reduce the need for RBC transfusions.
- The recommended starting dose for adult patients is 50 to 100 Units/kg 3 times weekly intravenously or subcutaneously.
Zidovudine-treated HIV-infected Patients
- Epogen is indicated for the treatment of anemia due to zidovudine administered at ≤ 4200 mg/week in HIV-infected patients with endogenous serum erythropoietin levels of ≤ 500 mUnits/mL.
- Starting Dose
- The recommended starting dose in adults is 100 Units/kg as an intravenous or subcutaneous injection 3 times per week.
- Dose Adjustment
- If hemoglobin does not increase after 8 weeks of therapy, increase Epogen dose by approximately
- 50 to 100 Units/kg at 4- to 8-week intervals until hemoglobin reaches a level needed to avoid RBC transfusions or 300 Units/kg.
- Withhold Epogen if hemoglobin exceeds 12 g/dL. Resume therapy at a dose 25% below the previous dose when hemoglobin declines to less than 11 g/dL.
Discontinue Epogen if an increase in hemoglobin is not achieved at a dose of 300 Units/kg for 8 weeks.
Patients on Cancer Chemotherapy
- Epogen is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
- Initiate Epogen in patients on cancer chemotherapy only if the hemoglobin is less than 10 g/dL, and if there is a minimum of two additional months of planned chemotherapy.
- Use the lowest dose of Epogen necessary to avoid RBC transfusions.
- Recommended Starting Dose
- Adults:
- 150 Units/kg subcutaneously 3 times per week until completion of a chemotherapy course or
- 40,000 Units subcutaneously weekly until completion of a chemotherapy course.
- Dose Reduction
- Reduce dose by 25% if:
- Hemoglobin increases greater than 1 g/dL in any 2-week period or
- Hemoglobin reaches a level needed to avoid RBC transfusion.
- Withhold dose if hemoglobin exceeds a level needed to avoid RBC transfusion. Reinitiate at a dose 25% below the previous dose when hemoglobin approaches a level where RBC transfusions may be required.
- Dose Increase
- After the initial 4 weeks of Epogen therapy, if hemoglobin increases by less than 1 g/dL and remains below 10 g/dL, increase dose to:
- 300 Units/kg three times per week in adults or
- 60,000 Units weekly in adults
- 900 Units/kg (maximum 60,000 Units) weekly in children
- After 8 weeks of therapy, if there is no response as measured by hemoglobin levels or if RBC transfusions are still required, discontinue Epogen.
Surgery Patients
- Epogen is indicated to reduce the need for allogeneic RBC transfusions among patients with perioperative hemoglobin > 10 to ≤ 13 g/dL who are at high risk for perioperative blood loss from elective, noncardiac, nonvascular surgery. Epogen is not indicated for patients who are willing to donate autologous blood pre-operatively.
- 300 Units/kg per day subcutaneously for 15 days total: administered daily for 10 days before surgery, on the day of surgery, and for 4 days after surgery.
- 600 Units/kg subcutaneously in 4 doses administered 21, 14, and 7 days before surgery and on the day of surgery.
- Deep venous thrombosis prophylaxis is recommended during Epogen therapy.
Preparation and Administration
- Do not shake. Do not use Epogen that has been shaken or frozen.
- Protect vials from light.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use any vials exhibiting particulate matter or discoloration.
- Discard unused portions of Epogen in preservative-free vials. Do not re-enter preservative-free vials.
- Store unused portions of Epogen in multidose vials at 36°F to 46° F (2°C to 8°C). Discard 21 days after initial entry.
- Do not dilute. Do not mix with other drug solutions except for admixing as described below:
- Preservative-free Epogen from single-use vials may be admixed in a syringe with bacteriostatic 0.9% sodium chloride injection, USP, with benzyl alcohol 0.9% (bacteriostatic saline) in a 1:1 ratio using aseptic technique at the time of administration. Risks are associated with benzyl alcohol in neonates, infants, pregnant women, and nursing mothers.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Epoetin alfa in adult patients.
Non–Guideline-Supported Use
Anemia - Congestive heart failure
- Subcutaneous erythropoietin (average dose: 5227 units/week) and intravenous (IV) iron (average dose: 185 milligrams (mg)/month).[1]
Anemia due to radiation
- Epoetin alfa 200 units/kilogram/day for 5 consecutive days per week for up to 7 weeks during radiotherapy.
Anemia during the puerperium
- Intravenous (IV) erythropoietin (EPO) 300 units/kilogram/day plus IV iron sucrose 200 milligrams (mg)/day on days 1 to 4 postpartum.[2]
Anemia - Hepatitis C, In patients being treated with a combination of ribavirin and interferon alfa or ribavirin and peginterferon alfa
- Epoetin alfa (Procrit(R)) 40,000 units subcutaneously once weekly.[3]
Anemia - Multiple myeloma
- Erythropoietin (150 units/kilogram 3 times/week initially with adjustments every 3 weeks as needed).
Anemia - Myelodysplastic syndrome
- Erythropoietin alfa 150 international units/kilogram subcutaneously 3 times weekly for 26 weeks.[4]
Anemia - Myelofibrosis
- Subcutaneous erythropoietin 10,000 units 3 days per week.[5]
Anemia - Rheumatoid arthritis
- 100 units/kilogram (kg) 3 times per week for eight weeks.
- 150 international units/kilogram subcutaneously was administered 3 times per week for at least 12 weeks.[6]
Blood unit collection for autotransfusion
- Epoetin alfa doses of 12,000 to 24,000 units subcutaneously once weekly or 300 to 600 units/kilogram twice weekly.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Patients on Cancer Chemotherapy
- Epogen is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy
- Pediatric Patients (5 to 18 years):
- 600 Units/kg intravenously weekly until completion of a chemotherapy course.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Epoetin alfa in pediatric patients.
Non–Guideline-Supported Use
Anemia of prematurity
- IV erythropoietin dosing was 1250 units/kg/wk as 5 divided doses.
Contraindications
- Uncontrolled hypertension.
- Pure red cell aplasia (PRCA) that begins after treatment with Epogen or other erythropoietin protein drugs.
- Serious allergic reactions to Epogen.
- Epogen from multidose vials contains benzyl alcohol and is contraindicated in:
- Neonates, infants, pregnant women, and nursing mothers. Benzyl alcohol has been associated with serious adverse events and death, particularly in pediatric patients. When therapy with Epogen is needed in neonates and infants, use single-dose vials; do not admix with bacteriostatic saline containing benzyl alcohol.
Warnings
|
ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
See full prescribing information for complete Boxed Warning.
|
Precautions
Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- In controlled clinical trials of patients with CKD comparing higher hemoglobin targets (13 - 14 g/dL) to lower targets (9 - 11.3 g/dL), Epogen and other ESAs increased the risk of death, myocardial infarction, stroke, congestive heart failure, thrombosis of hemodialysis vascular access, and other thromboembolic events in the higher target groups.
- Using ESAs to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit. Use caution in patients with coexistent cardiovascular disease and stroke. Patients with CKD and an insufficient hemoglobin response to ESA therapy may be at even greater risk for cardiovascular reactions and mortality than other patients. A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks.
- In controlled clinical trials of patients with cancer, Epogen and other ESAs increased the risks for death and serious adverse cardiovascular reactions. These adverse reactions included myocardial infarction and stroke.
- In controlled clinical trials, ESAs increased the risk of death in patients undergoing coronary artery bypass graft surgery (CABG) and the risk of deep venous thrombosis (DVT) in patients undergoing orthopedic procedures.
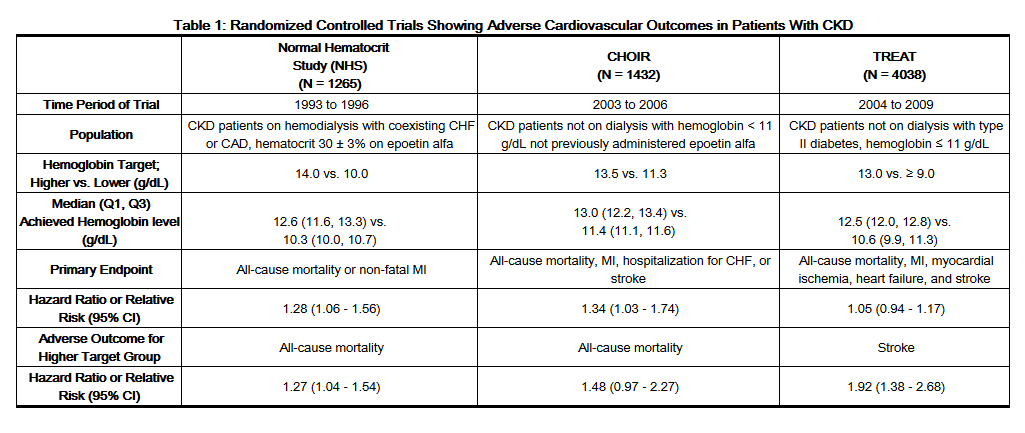
- The design and overall results of the 3 large trials comparing higher and lower hemoglobin targets are shown in Table 1.
- Patients with Chronic Kidney Disease
- Normal Hematocrit Study (NHS)
- A prospective, randomized, open-label study of 1265 patients with chronic kidney disease on dialysis with documented evidence of congestive heart failure or ischemic heart disease was designed to test the hypothesis that a higher target hematocrit (Hct) would result in improved outcomes compared with a lower target Hct. In this study, patients were randomized to epoetin alfa treatment targeted to a maintenance hemoglobin of either 14 ± 1 g/dL or 10 ± 1 g/dL. The trial was terminated early with adverse safety findings of higher mortality in the high hematocrit target group. Higher mortality (35% vs. 29%) was observed for the patients randomized to a target hemoglobin of 14 g/dL than for the patients randomized to a target hemoglobin of 10 g/dL. For all-cause mortality, the HR=1.27; 95% CI (1.04, 1.54); p=0.018. The incidence of nonfatal myocardial infarction, vascular access thrombosis, and other thrombotic events was also higher in the group randomized to a target hemoglobin of 14 g/dL.
- CHOIR
- A randomized, prospective trial, 1432 patients with anemia due to CKD who were not undergoing dialysis and who had not previously received epoetin alfa therapy were randomized to epoetin alfa treatment targeting a maintenance hemoglobin concentration of either 13.5 g/dL or 11.3 g/dL. The trial was terminated early with adverse safety findings. A major cardiovascular event (death, myocardial infarction, stroke, or hospitalization for congestive heart failure) occurred in 125 of the 715 patients (18%) in the higher hemoglobin group compared to 97 of the 717 patients (14%) in the lower hemoglobin group [hazard ratio (HR) 1.34, 95% CI: 1.03, 1.74; p = 0.03].
- TREAT
- A randomized, double-blind, placebo-controlled, prospective trial of 4038 patients with: CKD not on dialysis (eGFR of 20 – 60 mL/min), anemia (hemoglobin levels ≤ 11 g/dL), and type 2 diabetes mellitus, patients were randomized to receive either darbepoetin alfa treatment or a matching placebo. Placebo group patients also received darbepoetin alfa when their hemoglobin levels were below 9 g/dL. The trial objectives were to demonstrate the benefit of darbepoetin alfa treatment of the anemia to a target hemoglobin level of 13 g/dL, when compared to a "placebo" group, by reducing the occurrence of either of two primary endpoints: (1) a composite cardiovascular endpoint of all-cause mortality or a specified cardiovascular event (myocardial ischemia, CHF,MI, and CVA) or (2) a composite renal endpoint of all-cause mortality or progression to end stage renal disease. The overall risks for each of the two primary endpoints (the cardiovascular composite and the renal composite) were not reduced with darbepoetin alfa treatment (see Table 1), but the risk of stroke was increased nearly two-fold in the darbepoetin alfa -treated group versus the placebo group: annualized stroke rate 2.1% vs. 1.1%, respectively, HR 1.92; 95% CI: 1.38, 2.68; p < 0.001. The relative risk of stroke was particularly high in patients with a prior stroke: annualized stroke rate 5.2% in the darbepoetin alfa- treated group and 1.9% in the placebo group, HR 3.07; 95% CI: 1.44, 6.54. Also, among darbepoetin alfa -treated subjects with a past history of cancer, there were more deaths due to all causes and more deaths adjudicated as due to cancer, in comparison with the control group.
- Normal Hematocrit Study (NHS)
- Patients with Cancer
- An increased incidence of thromboembolic reactions, some serious and life-threatening, occurred in patients with cancer treated with ESAs.
- In a randomized, placebo-controlled study (Study 1 in Table 2) of 939 women with metastatic breast cancer receiving chemotherapy, patients received either weekly epoetin alfa or placebo for up to a year. This study was designed to show that survival was superior when epoetin alfa was administered to prevent anemia (maintain hemoglobin levels between 12 and 14 g/dL or hematocrit between 36% and 42%). This study was terminated prematurely when interim results demonstrated a higher mortality at 4 months (8.7% vs. 3.4%) and a higher rate of fatal thrombotic reactions (1.1% vs. 0.2%) in the first 4 months of the study among patients treated with epoetin alfa. Based on Kaplan-Meier estimates, at the time of study termination, the 12-month survival was lower in the epoetin alfa group than in the placebo group (70% vs. 76%; HR 1.37, 95% CI: 1.07, 1.75; p = 0.012).
- Patients Having Surgery
- An increased incidence of deep venous thrombosis (DVT) in patients receiving epoetin alfa undergoing surgical orthopedic procedures was demonstrated. In a randomized, controlled study, 680 adult patients, not receiving prophylactic anticoagulation and undergoing spinal surgery, were randomized to 4 doses of 600 Units/kg epoetin alfa (7, 14, and 21 days before surgery, and the day of surgery) and standard of care (SOC) treatment (n = 340) or to SOC treatment alone (n = 340). A higher incidence of DVTs, determined by either color flow duplex imaging or by clinical symptoms, was observed in the epoetin alfa group (16 [4.7%] patients) compared with the SOC group (7 [2.1%] patients). In addition to the 23 patients with DVTs included in the primary analysis, 19 [2.8%] patients (n = 680) experienced 1 other thrombovascular event (TVE) each (12 [3.5%] in the epoetin alfa group and 7 [2.1%] in the SOC group). Deep venous thrombosis prophylaxis is strongly recommended when ESAs are used for the reduction of allogeneic RBC transfusions in surgical patients.
- Increased mortality was observed in a randomized, placebo-controlled study of Epogen in adult patients who were undergoing CABG surgery (7 deaths in 126 patients randomized to Epogen versus no deaths among 56 patients receiving placebo). Four of these deaths occurred during the period of study drug administration and all 4 deaths were associated with thrombotic events.
Prescribing and Distribution Program for Epogen in Patients With Cancer
- In order to prescribe and/or dispense Epogen to patients with cancer and anemia due to myelosuppressive chemotherapy, prescribers and hospitals must enroll in and comply with the ESA APPRISE Oncology Program requirements. To enroll, visit www.esa-apprise.com or call 1-866-284-8089 for further assistance. Additionally, prior to each new course of Epogen in patients with cancer, prescribers and patients must provide written acknowledgment of a discussion of the risks of Epogen.
Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients With Cancer
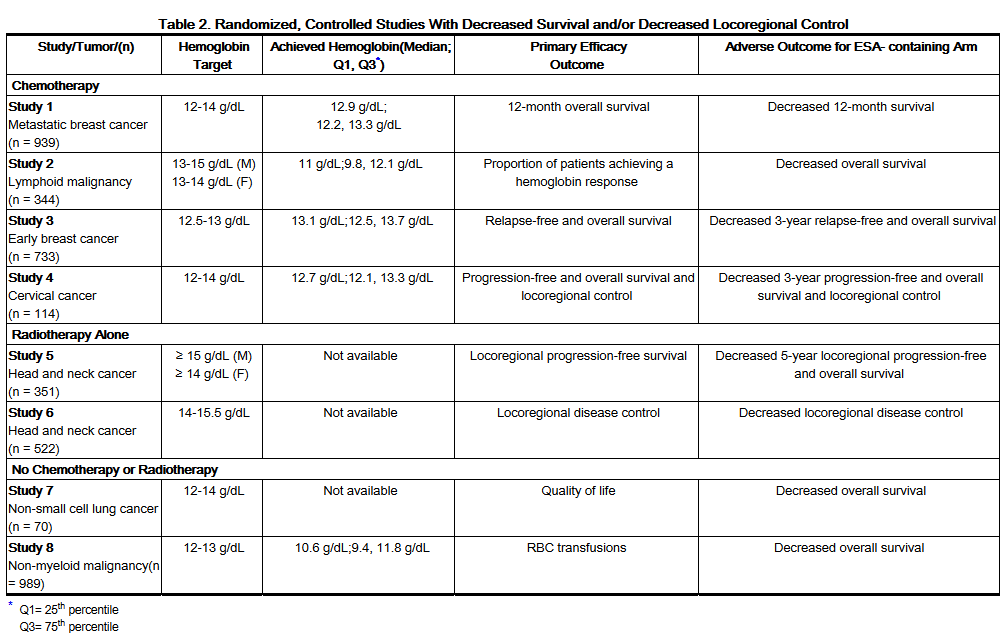
- ESAs resulted in decreased locoregional control/progression-free survival and/or overall survival (see Table 2). These findings were observed in studies of patients with advanced head and neck cancer receiving radiation therapy (Studies 5 and 6), in patients receiving chemotherapy for metastatic breast cancer (Study 1) or lymphoid malignancy (Study 2), and in patients with non-small cell lung cancer or various malignancies who were not receiving chemotherapy or radiotherapy (Studies 7 and 8).
- Decreased Overall Survival
- Study 1 was described in the previous section. Mortality at 4 months (8.7% vs. 3.4%) was significantly higher in the epoetin alfa arm. The most common investigator-attributed cause of death within the first 4 months was disease progression; 28 of 41 deaths in the epoetin alfa arm and 13 of 16 deaths in the placebo arm were attributed to disease progression. Investigator-assessed time to tumor progression was not different between the 2 groups. Survival at 12 months was significantly lower in the epoetin alfa arm (70% vs. 76%; HR 1.37, 95% CI: 1.07, 1.75; p = 0.012).
- Study 2 was a randomized, double-blind study (darbepoetin alfa vs. placebo) conducted in 344 anemic patients with lymphoid malignancy receiving chemotherapy. With a median follow-up of 29 months, overall mortality rates were significantly higher among patients randomized to darbepoetin alfa as compared to placebo (HR 1.36, 95% CI: 1.02, 1.82).
- Study 7 was a multicenter, randomized, double-blind study (epoetin alfa vs. placebo) in which patients with advanced non-small cell lung cancer receiving only palliative radiotherapy or no active therapy were treated with epoetin alfa to achieve and maintain hemoglobin levels between 12 and 14 g/dL. Following an interim analysis of 70 patients (planned accrual 300 patients), a significant difference in survival in favor of the patients in the placebo arm of the study was observed (median survival 63 vs. 129 days; HR 1.84; p = 0.04).
- Study 8 was a randomized, double-blind study (darbepoetin alfa vs. placebo) in 989 anemic patients with active malignant disease, neither receiving nor planning to receive chemotherapy or radiation therapy. There was no evidence of a statistically significant reduction in proportion of patients receiving RBC transfusions. The median survival was shorter in the darbepoetin alfa treatment group than in the placebo group (8 months vs. 10.8 months; HR 1.30, 95% CI: 1.07, 1.57).
- Decreased Progression-free Survival and Overall Survival
- Study 3 was a randomized, open-label, controlled, factorial design study in which darbepoetin alfa was administered to prevent anemia in 733 women receiving neo-adjuvant breast cancer treatment. A final analysis was performed after a median follow-up of approximately 3 years. The 3-year survival rate was lower (86% vs. 90%; HR 1.42, 95% CI: 0.93, 2.18) and the 3-year relapse-free survival rate was lower (72% vs. 78%; HR 1.33, 95% CI: 0.99, 1.79) in the darbepoetin alfa-treated arm compared to the control arm.
- Study 4 was a randomized, open-label, controlled study that enrolled 114 of a planned 460 cervical cancer patients receiving chemotherapy and radiotherapy. Patients were randomized to receive epoetin alfa to maintain hemoglobin between 12 and 14 g/dL or to RBC transfusion support as needed. The study was terminated prematurely due to an increase in thromboembolic adverse reactions in epoetin alfa-treated patients compared to control (19% vs. 9%). Both local recurrence (21% vs. 20%) and distant recurrence (12% vs. 7%) were more frequent in epoetin alfa-treated patients compared to control. Progression-free survival at 3 years was lower in the epoetin alfa-treated group compared to control (59% vs. 62%; HR 1.06, 95% CI: 0.58, 1.91). Overall survival at 3 years was lower in the epoetin alfa-treated group compared to control (61% vs. 71%; HR 1.28, 95% CI: 0.68, 2.42).
- Study 5 was a randomized, placebo-controlled study in 351 head and neck cancer patients where epoetin beta or placebo was administered to achieve target hemoglobins ≥ 14 and ≥ 15 g/dL for women and men, respectively. Locoregional progression-free survival was significantly shorter in patients receiving epoetin beta (HR 1.62, 95% CI: 1.22, 2.14; p = 0.0008) with medians of 406 days and 745 days in the epoetin beta and placebo arms, respectively. Overall survival was significantly shorter in patients receiving epoetin beta (HR 1.39, 95% CI: 1.05, 1.84; p = 0.02).
- Decreased Locoregional Control
- Study 6 was a randomized, open-label, controlled study conducted in 522 patients with primary squamous cell carcinoma of the head and neck receiving radiation therapy alone (no chemotherapy) who were randomized to receive darbepoetin alfa to maintain hemoglobin levels of 14 to15.5 g/dL or no darbepoetin alfa. An interim analysis performed on 484 patients demonstrated that locoregional control at 5 years was significantly shorter in patients receiving darbepoetin alfa (RR 1.44, 95% CI: 1.06, 1.96; p = 0.02). Overall survival was shorter in patients receiving darbepoetin alfa (RR 1.28, 95% CI: 0.98, 1.68; p = 0.08).
Hypertension
- Epogen is contraindicated in patients with uncontrolled hypertension. Following initiation and titration of Epogen, approximately 25% of patients on dialysis required initiation of or increases in antihypertensive therapy; hypertensive encephalopathy and seizures have been reported in patients with CKD receiving Epogen.
- Appropriately control hypertension prior to initiation of and during treatment with Epogen. Reduce or withhold Epogen if blood pressure becomes difficult to control. Advise patients of the importance of compliance with antihypertensive therapy and dietary restrictions.
Seizures
- Epogen increases the risk of seizures in patients with CKD. During the first several months following initiation of Epogen, monitor patients closely for premonitory neurologic symptoms. Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms or change in seizure frequency.
Lack or Loss of Hemoglobin Response to Epogen
- For lack or loss of hemoglobin response to Epogen, initiate a search for causative factors (e.g., iron deficiency, infection, inflammation, bleeding). If typical causes of lack or loss of hemoglobin response are excluded, evaluate for PRCA. In the absence of PRCA, follow dosing recommendations for management of patients with an insufficient hemoglobin response to Epogen therapy.
Pure Red Cell Aplasia
- Cases of PRCA and of severe anemia, with or without other cytopenias that arise following the development of neutralizing antibodies to erythropoietin have been reported in patients treated with Epogen. This has been reported predominantly in patients with CKD receiving ESAs by subcutaneous administration. PRCA has also been reported in patients receiving ESAs for anemia related to hepatitis C treatment (an indication for which Epogen is not approved).
- If severe anemia and low reticulocyte count develop during treatment with Epogen, withhold Epogen and evaluate patients for neutralizing antibodies to erythropoietin. Contact Amgen (1-800-77-AMGEN) to perform assays for binding and neutralizing antibodies. Permanently discontinue Epogen in patients who develop PRCA following treatment with Epogen or other erythropoietin protein drugs. Do not switch patients to other ESAs.
Serious Allergic Reactions
- Serious allergic reactions, including anaphylactic reactions, angioedema, bronchospasm, skin rash, and urticaria may occur with Epogen. Immediately and permanently discontinue Epogen and administer appropriate therapy if a serious allergic or anaphylactic reaction occurs.
Albumin (Human)
- Epogen contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Dialysis Management
- Patients may require adjustments in their dialysis prescriptions after initiation of Epogen. Patients receiving Epogen may require increased anticoagulation with heparin to prevent clotting of the extracorporeal circuit during hemodialysis.
Laboratory Monitoring
- Evaluate transferrin saturation and serum ferritin prior to and during Epogen treatment. Administer supplemental iron therapy when serum ferritin is less than 100 mcg/L or when serum transferrin saturation is less than 20%. The majority of patients with CKD will require supplemental iron during the course of ESA therapy. Following initiation of therapy and after each dose adjustment, monitor hemoglobin weekly until the hemoglobin level is stable and sufficient to minimize the need for RBC transfusion.
Adverse Reactions
Clinical Trials Experience
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- Increased mortality and/or increased risk of tumor progression or recurrence in Patients With Cancer
- Hypertension
- Seizures
- PRCA
- Serious allergic reactions.
Patients with Chronic Kidney Disease
- Adult Patients
- Three double-blind, placebo-controlled studies, including 244 patients with CKD on dialysis, were used to identify the adverse reactions to Epogen. In these studies, the mean age of patients was 48 years (range: 20 to 80 years). One hundred and thirty-three (55%) patients were men. The racial distribution was as follows: 177 (73%) patients were white, 48 (20%) patients were black, 4 (2%) patients were Asian, 12 (5%) patients were other, and racial information was missing for 3 (1%) patients.
- Two double-blind, placebo-controlled studies, including 210 patients with CKD not on dialysis, were used to identify the adverse reactions to Epogen. In these studies, the mean age of patients was 57 years (range: 24 to 79 years). One hundred and twenty-one (58%) patients were men. The racial distribution was as follows: 164 (78%) patients were white, 38 (18%) patients were black, 3 (1%) patients were Asian, 3 (1%) patients were other, and racial information was missing for 2 (1%) patients.
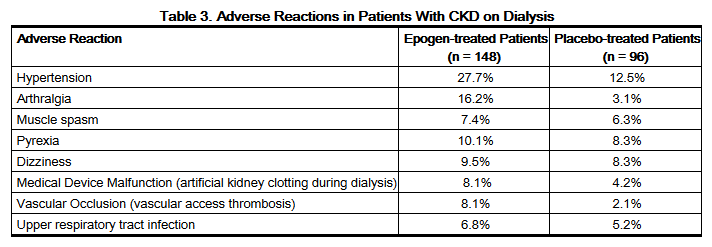
- The adverse reactions with a reported incidence of ≥ 5% in Epogen-treated patients and that occurred at a ≥ 1% higher frequency than in placebo-treated patients are shown in the table below:
- An additional serious adverse reaction that occurred in less than 5% of epoetin alfa-treated dialysis patients and greater than placebo was thrombosis (2.7% Epogen and 1% placebo).
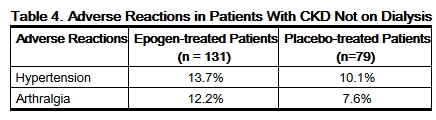
- The adverse reactions with a reported incidence of ≥ 5% in Epogen-treated patients and that occurred at a ≥ 1% higher frequency than in placebo-treated patients are shown in the table below:
- Additional serious adverse reactions that occurred in less than 5% of epoetin alfa-treated patients not on dialysis and greater than placebo were erythema (0.8% Epogen and 0% placebo) and myocardial infarction (0.8% Epogen and 0% placebo).
- Pediatric Patients
- In pediatric patients with CKD on dialysis, the pattern of adverse reactions was similar to that found in adults.
Zidovudine-treated HIV-infected Patients
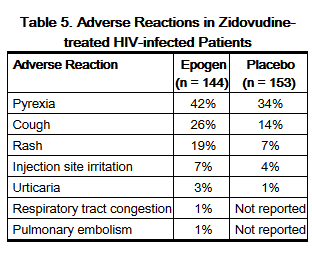
- A total of 297 zidovudine-treated HIV-infected patients were studied in 4 placebo-controlled studies. A total of 144 (48%) patients were randomly assigned to receive Epogen and 153 (52%) patients were randomly assigned to receive placebo. Epogen was administered at doses between 100 and 200 Units/kg 3 times weekly subcutaneously for up to 12 weeks.
- For the combined Epogen treatment groups, a total of 141 (98%) men and 3 (2%) women between the ages of 24 and 64 years were enrolled. The racial distribution of the combined Epogen treatment groups was as follows: 129 (90%) white, 8 (6%) black, 1 (1%) Asian, and 6 (4%) other.
- In double-blind, placebo-controlled studies of 3 months duration involving approximately 300 zidovudine-treated HIV-infected patients, adverse reactions with an incidence of ≥ 1% in patients treated with Epogen were:
Cancer Patients on Chemotherapy
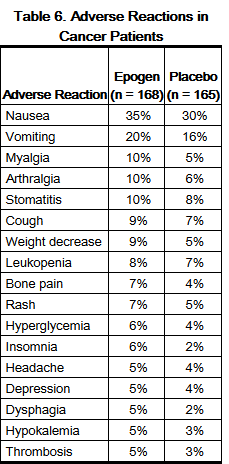
- The data below were obtained in Study C1, a 16-week, double-blind, placebo-controlled study that enrolled 344 patients with anemia secondary to chemotherapy. There were 333 patients who were evaluable for safety; 168 of 174 patients (97%) randomized to Epogen received at least 1 dose of study drug, and 165 of 170 patients (97%) randomized to placebo received at least 1 placebo dose. For the once weekly Epogen-treatment group, a total of 76 men (45%) and 92 women (55%) between the ages of 20 and 88 years were treated. The racial distribution of the Epogen-treatment group was 158 white (94%) and 10 black (6%). Epogen was administered once weekly for an average of 13 weeks at a dose of 20,000 to 60,000 IU subcutaneously (mean weekly dose was 49,000 IU).
- The adverse reactions with a reported incidence of ≥ 5% in Epogen-treated patients that occurred at a higher frequency than in placebo-treated patients are shown in the table below:
Surgery Patients
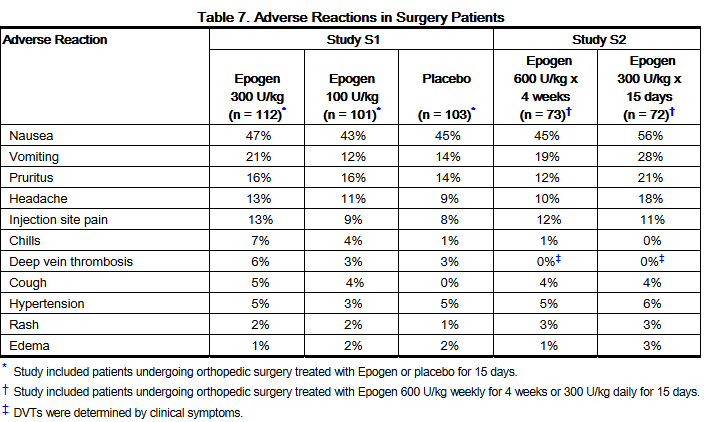
- Four hundred sixty-one patients undergoing major orthopedic surgery were studied in a placebo-controlled study (S1) and a comparative dosing study (2 dosing regimens, S2). A total of 358 patients were randomly assigned to receive Epogen and 103 (22%) patients were randomly assigned to receive placebo. Epogen was administered daily at a dose of 100 to 300 IU/kg subcutaneously for 15 days or at 600 IU/kg once weekly for 4 weeks.
- For the combined Epogen treatment groups, a total of 90 (25%) and 268 (75%) women between the ages of 29 and 89 years were enrolled. The racial distribution of the combined Epogen treatment groups was as follows: 288 (80%) white, 64 (18%) black, 1 (< 1%) Asian, and 5 (1%) other.
- The adverse reactions with a reported incidence of ≥ 1% in Epogen-treated patients that occurred at a higher frequency than in placebo-treated patients are shown in the table below:
Postmarketing Experience
- Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The following adverse reactions have been identified during postmarketing use of Epogen:
- Seizures
- PRCA
- Serious allergic reactions
- Injection site reactions, including irritation and pain
- Porphyria
Drug Interactions
- No formal drug interaction studies have been conducted with Epogen.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well-controlled studies of Epogen use during pregnancy. There are limited data on Epogen use in pregnant women. In animal reproductive and developmental toxicity studies, adverse fetal effects occurred when pregnant rats received epoetin alfa at doses approximating the clinical recommended starting doses. Single-dose formulations of Epogen should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- There are reports of at least 33 pregnant women with anemia alone or anemia associated with severe renal disease and other hematologic disorders who received Epogen. Polyhydramnios and intrauterine growth restriction were reported in women with chronic renal disease, which is associated with an increased risk for these adverse pregnancy outcomes. There was 1 infant born with pectus excavatum and hypospadias following exposure during the first trimester. Due to the limited number of exposed pregnancies and multiple confounding factors (such as underlying maternal conditions, other maternal medications, and gestational timing of exposure), these published case reports and studies do not reliably estimate the frequency or absence of adverse outcomes.
- When healthy rats received Epogen at doses of 100 Units/kg/day during mating and through early pregnancy (dosing stopped prior to organogenesis), there were slight increases in the incidences of pre-and post-implantation loss, and a decrease in live fetuses. This animal dose level of 100 Units/kg/day may approximate the clinical recommended starting dose, depending on the treatment indication. When healthy pregnant rats and rabbits received intravenous doses of up to 500 mg/kg/day of Epogen only during organogenesis, no teratogenic effects were observed in the offspring.
- When healthy pregnant rats received Epogen at doses of 500 Units/kg/day late in pregnancy (after the period of organogenesis), offspring had decreased number of caudal vertebrae and growth delays.
- Women who become pregnant during Epogen treatment are encouraged to enroll in Amgen’s Pregnancy Surveillance Program. Patients or their physicians should call 1-800-772-6436 (1-800-77-AMGEN) to enroll.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Epoetin alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Epoetin alfa during labor and delivery.
Nursing Mothers
- The multidose vials of Epogen are formulated with benzyl alcohol. Do not administer Epogen from multidose vials, or Epogen from single-dose vials admixed with bacteriostatic saline containing benzyl alcohol, to a nursing woman. When therapy with Epogen is needed in nursing women, use a benzyl alcohol-free formulation.
- It is not known whether Epogen is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Epogen from single-dose vials is administered to a nursing woman.
Pediatric Use
- The multidose vials are formulated with benzyl alcohol. Do not administer Epogen from multidose vials, or Epogen from single-dose vials admixed with bacteriostatic saline containing benzyl alcohol, to neonates or infants. When therapy with Epogen is needed in neonates and infants, use a benzyl alcohol-free formulation.
- Benzyl alcohol has been associated with serious adverse events and death, particularly in pediatric patients. The "gasping syndrome," (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages > 99 mg/kg/day in neonates and low-birthweight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse.
- Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the "gasping syndrome", the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birthweight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
- Pediatric Patients on Dialysis
- Epogen is indicated in pediatric patients, ages 1 month to 16 years of age, for the treatment of anemia associated with CKD requiring dialysis. Safety and effectiveness in pediatric patients less than 1 month old have not been established.
- The safety data from these studies are similar to those obtained from the studies of Epogen in adult patients with CKD.
- Pediatric Cancer Patients on Chemotherapy
- Epogen is indicated in patients 5 to 18 years old for the treatment of anemia due to concomitant myelosuppressive chemotherapy. Safety and effectiveness in pediatric patients less than 5 years of age have not been established. The safety data from these studies are similar to those obtained from the studies of Epogen in adult patients with cancer.
- Pediatric Patients With HIV Infection Receiving Zidovudine
- Published literature has reported the use of Epogen in 20 zidovudine-treated, anemic, pediatric patients with HIV infection, ages 8 months to 17 years, treated with 50 to 400 Units/kg subcutaneously or intravenously 2 to 3 times per week. Increases in hemoglobin levels and in reticulocyte counts and decreases in or elimination of RBC transfusions were observed.
- Pharmacokinetics in Neonates
- Limited pharmacokinetic data from a study of 7 preterm, very low birth weight neonates and 10 healthy adults given intravenous erythropoietin suggested that distribution volume was approximately 1.5 to 2 times higher in the preterm neonates than in the healthy adults, and clearance was approximately 3 times higher in the preterm neonates than in the healthy adults.
Geriatic Use
- Of the 4553 patients who received Epogen in the 6 studies for treatment of anemia due to CKD not receiving dialysis, 2726 (60%) were age 65 years and over, while 1418 (31%) were 75 years and over. Of the 757 patients who received Epogen in the 3 studies of CKD patients on dialysis, 361 (47%) were age 65 years and over, while 100 (13%) were 75 years and over. No differences in safety or effectiveness were observed between geriatric and younger patients. Dose selection and adjustment for an elderly patient should be individualized to achieve and maintain the target hemoglobin.
- Among 778 patients enrolled in the 3 clinical studies of Epogen for the treatment of anemia due to concomitant chemotherapy, 419 received Epogen and 359 received placebo. Of the 419 who received Epogen, 247 (59%) were age 65 years and over, while 78 (19%) were 75 years and over. No overall differences in safety or effectiveness were observed between geriatric and younger patients. The dose requirements for Epogen in geriatric and younger patients within the 3 studies were similar.
- Among 1731 patients enrolled in the 6 clinical studies of Epogen for reduction of allogeneic RBC transfusions in patients undergoing elective surgery, 1085 received Epogen and 646 received placebo or standard of care treatment. Of the 1085 patients who received Epogen, 582 (54%) were age 65 years and over, while 245 (23%) were 75 years and over. No overall differences in safety or effectiveness were observed between geriatric and younger patients. The dose requirements for Epogen in geriatric and younger patients within the 4 studies using the 3 times weekly schedule and 2 studies using the weekly schedule were similar.
- Insufficient numbers of patients age 65 years or older were enrolled in clinical studies of Epogen for the treatment of zidovudine in HIV-infected patients to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Epoetin alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Epoetin alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Epoetin alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Epoetin alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Epoetin alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Epoetin alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subcutaneous
- Intravenous
Monitoring
- To undergo regular blood pressure monitoring.
- Evaluate transferrin saturation and serum ferritin prior to and during Epogen treatment.
- Epogen increases the risk for seizures in patients with CKD. Increase monitoring of these patients for changes in seizure frequency or premonitory symptoms
IV Compatibility
There is limited information regarding IV Compatibility of Epoetin alfa in the drug label.
Overdosage
- Epogen overdosage can cause hemoglobin levels above the desired level, which should be managed with discontinuation or reduction of Epogen dosage and/or with phlebotomy as clinically indicated.
- Cases of severe hypertension have been observed following overdose with ESAs
Chronic Overdose
There is limited information regarding Chronic Overdose of Epoetin alfa in the drug label.
Pharmacology
Epoetin alfa
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | B03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 18396.1 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
Unknown |
| Legal status |
Prescription Only (S4)(AU) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV or subcutaneous |
Mechanism of Action
- Epogen stimulates erythropoiesis by the same mechanism as endogenous erythropoietin.
Structure
- Epogen (epoetin alfa) is a 165-amino acid erythropoiesis-stimulating glycoprotein manufactured by recombinant DNA technology. It has a molecular weight of approximately 30,400 daltons and is produced by mammalian cells into which the human erythropoietin gene has been introduced. The product contains the identical amino acid sequence of isolated natural erythropoietin.
- Epogen is formulated as a sterile, colorless liquid in vials in multiple formulations. Single-dose vials, formulated with an isotonic sodium chloride/sodium citrate-buffered solution, are supplied in multiple strengths. Each 1 mL vial contains 2000, 3000, 4000, or 10,000 Units of epoetin alfa, Albumin (Human) (2.5 mg), citric acid (0.06 mg), sodium chloride (5.9 mg), and sodium citrate (5.8 mg) in Water for Injection, USP (pH 6.9 ± 0.3). Single-dose 1 mL vials formulated with an isotonic sodium chloride/sodium phosphate buffer contain 40,000 Units of epoetin alfa albumin (human) (2.5 mg), citric acid (0.0068 mg), sodium chloride (5.8 mg), sodium citrate (0.7 mg), sodium phosphate dibasic anhydrate (1.8 mg), and sodium phosphate monobasic monohydrate (1.2 mg) in Water for Injection, USP (pH 6.9 ± 0.3). Multidose, 2 mL vials contain 10,000 Units epoetin alfa, albumin (human) (2.5 mg), benzyl alcohol (1%), sodium chloride (8.2 mg), and sodium citrate (1.3 mg) per 1 mL Water for Injection, USP (pH 6.1 ± 0.3). Multidose 1 mL vials contain 20,000 Units epoetin alfa, albumin (human) (2.5 mg), benzyl alcohol (1%), sodium chloride (8.2 mg), citric acid (0.11 mg), and sodium citrate (1.3 mg), per 1 mL in Water for Injection, USP (pH 6.1 ± 0.3).
Pharmacodynamics
- Epogen increases the reticulocyte count within 10 days of initiation, followed by increases in the RBC count, hemoglobin, and hematocrit, usually within 2 to 6 weeks. The rate of hemoglobin increase varies among patients and is dependent upon the dose of Epogen administered. For correction of anemia in hemodialysis patients, a greater biologic response is not observed at doses exceeding 300 Units/kg 3 times weekly.
Pharmacokinetics
- In adult and pediatric patients with CKD, the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of Epogen ranged from 4 to 13 hours. After subcutaneous administration, Cmax was achieved within 5 to 24 hours. The t1/2 in adult patients with serum creatinine greater than 3 mg/dL was similar between those not on dialysis and those maintained on dialysis. The pharmacokinetic data indicate no apparent difference in Epogen t1/2 among adult patients above or below 65 years of age.
- A pharmacokinetic study comparing 150 Units/kg subcutaneous 3 times weekly to 40,000 Units subcutaneous weekly dosing regimen was conducted for 4 weeks in healthy subjects (n = 12) and for 6 weeks in anemic cancer patients (n = 32) receiving cyclic chemotherapy. There was no accumulation of serum erythropoietin after the 2 dosing regimens during the study period. The 40,000 Units weekly regimen had a higher Cmax (3- to 7-fold), longer Tmax (2- to 3-fold), higher AUC0-168 h (2- to 3-fold) of erythropoietin and lower clearance (CL) (50%) than the 150 Units/kg 3 times weekly regimen. In anemic cancer patients, the average t1/2 was similar (40 hours with range of 16 to 67 hours) after both dosing regimens. After the 150 Units/kg 3 times weekly dosing, the values of Tmax and CL were similar (13.3 ± 12.4 vs. 14.2 ± 6.7 hours, and 20.2 ± 15.9 vs. 23.6 ± 9.5 mL/hr/kg) between week 1 when patients were receiving chemotherapy (n = 14) and week 3 when patients were not receiving chemotherapy (n = 4). Differences were observed after the 40,000 Units weekly dosing with longer Tmax (38 ± 18 hours) and lower CL (9.2 ± 4.7 mL/hr/kg) during week 1 when patients were receiving chemotherapy (n = 18) compared with those (22 ± 4.5 hours, 13.9 ± 7.6 mL/hr/kg, respectively) during week 3 when patients were not receiving chemotherapy (n = 7).
- The pharmacokinetic profile of Epogen in children and adolescents appeared similar to that of adults.
- The pharmacokinetics of Epogen has not been studied in patients with HIV infection.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity
- The carcinogenic potential of Epogen has not been evaluated.
- Mutagenicity
- Epogen was not mutagenic or clastogenic under the conditions tested: Epogen was negative in the in vitro bacterial reverse mutation assay (Ames test), in the in vitro mammalian cell gene mutation assay (the hypoxanthine-guanine phosphoribosyl transferase [HGPRT] locus), in an in vitro chromosomal aberration assay in mammalian cells, and in the in vivo mouse micronucleus assay.
- Impairment of Fertility
- When administered intravenously to male and female rats prior to and during mating, and to females through the beginning of implantation (up to gestational day 7; dosing stopped prior to the beginning of organogenesis), doses of 100 and 500 Units/kg/day of Epogen caused slight increases in pre-implantation loss, post-implantation loss and decreases in the incidence of live fetuses. It is not clear whether these effects reflect a drug effect on the uterine environment or on the conceptus. This animal dose level of 100 Units/kg/day approximates the clinical recommended starting dose, depending on the patient’s treatment indication, but may be lower than the clinical dose in patients whose doses have been adjusted.
Reproductive and Developmental Toxicology
- When pregnant rats were administered intravenous Epogen, 500 Units/kg/day, after the period of organogenesis (from day 17 of gestation through day 21 of lactation), their pups exhibited decreased number of caudal vertebrae, decreased body weight gain, and delayed appearance of abdominal hair, eyelid opening, and ossification. This animal dose level of 500 Units/kg/day is approximately 5-fold higher than the clinical recommended starting dose, depending on the patient’s treatment indication.
- When Epogen was administered intravenously during the period of organogenesis to pregnant rats (gestational days 7 to 17) and pregnant rabbits (gestational days 6 to 18), no evidence of teratogenic outcome was observed at the doses tested, up to 500 Units/kg/day. The offspring (F1 generation) of the treated rats were observed postnatally; rats from the F1 generation reached maturity and were mated; no Epogen-related effects were apparent for their offspring (F2 generation fetuses).
Clinical Studies
Patients With Chronic Kidney Disease
- Adult Patients on Dialysis
- Patients with chronic kidney disease on dialysis: ESA effects on rates of transfusion
- In clinical studies of CKD patients on dialysis, Epogen increased hemoglobin levels and decreased the need for RBC transfusion. Overall, more than 95% of patients were RBC transfusion-independent after receiving Epogen for 3 months. In clinical studies at starting doses of 50 to 150 Units/kg 3 times weekly, adult patients responded with an average rate of hemoglobin rise as presented in Table 8.
- The safety and efficacy of Epogen were evaluated in 13 clinical studies involving intravenous administration to a total of 1010 anemic patients on dialysis. Overall, more than 90% of the patients treated with Epogen experienced improvement in hemoglobin concentrations. In the 3 largest of these clinical studies, the median maintenance dose necessary to maintain the hemoglobin between 10 to 12 g/dL was approximately 75 Units/kg 3 times weekly. More than 95% of patients were able to avoid RBC transfusions. In the largest US multicenter study, approximately 65% of the patients received doses of 100 Units/kg 3 times weekly or less to maintain their hemoglobin at approximately 11.7 g/dL. Almost 10% of patients received a dose of 25 Units/kg or less, and approximately 10% received a dose of more than 200 Units/kg 3 times weekly to maintain their hemoglobin at this level.
- In the Normal Hematocrit Study, the yearly transfusion rate was 51.5% in the lower hemoglobin group (10 g/dL) and 32.4% in the higher hemoglobin group (14 g/dL).
- Other ESA trials
- In a 26-week, double-blind, placebo-controlled study, 118 patients on dialysis with an average hemoglobin of approximately 7 g/dL were randomized to either Epogen or placebo. By the end of the study, average hemoglobin increased to approximately 11 g/dL in the Epogen-treated patients and remained unchanged in patients receiving placebo. Epogen-treated patients experienced improvements in exercise tolerance and patient-reported physical functioning at month 2 that were maintained throughout the study.
- A multicenter, unit-dose study was also conducted in 119 patients receiving peritoneal dialysis who self-administered Epogen subcutaneously. Patients responded to Epogen administered subcutaneously in a manner similar to patients receiving intravenous administration.
Pediatric Patients on Dialysis
- The safety and efficacy of Epogen were studied in a placebo-controlled, randomized study of 113 children with anemia (hemoglobin ≤ 9 g/dL) undergoing peritoneal dialysis or hemodialysis. The initial dose of Epogen was 50 Units/kg intravenously or subcutaneously 3 times weekly. The dose of study drug was titrated to achieve either a hemoglobin of 10 to 12 g/dL or an absolute increase in hemoglobin of 2 g/dL over baseline.
- At the end of the initial 12 weeks, a statistically significant rise in mean hemoglobin (3.1 g/dL vs. 0.3 g/dL) was observed only in the Epogen arm. The proportion of children achieving a hemoglobin of 10 g/dL, or an increase in hemoglobin of 2 g/dL over baseline, at any time during the first 12 weeks was higher in the Epogen arm (96% vs. 58%). Within 12 weeks of initiating Epogen therapy, 92.3% of the pediatric patients were RBC transfusion independent as compared to 65.4% who received placebo. Among patients who received 36 weeks of Epogen, hemodialysis patients received a higher median maintenance dose [167 Units/kg/week (n = 28) vs. 76 Units/kg/week (n = 36)] and took longer to achieve a hemoglobin of 10 to 12 g/dL (median time to response 69 days vs. 32 days) than patients undergoing peritoneal dialysis.
Adult Patients With CKD Not Requiring Dialysis
- Four clinical studies were conducted in patients with CKD not on dialysis involving 181 patients treated with Epogen. These patients responded to Epogen therapy in a manner similar to that observed in patients on dialysis. Patients with CKD not on dialysis demonstrated a dose-dependent and sustained increase in hemoglobin when Epogen was administered by either an intravenous or subcutaneous route, with similar rates of rise of hemoglobin when Epogen was administered by either route.
Patients with chronic kidney disease not on dialysis: ESA effects on rates of transfusion
- In TREAT, a randomized, double-blind trial of 4038 patients with CKD and type 2 diabetes not on dialysis, a post-hoc analysis showed that the proportion of patients receiving RBC transfusions was lower in patients administered an ESA to target a hemoglobin of 13 g/dL compared to the control arm in which an ESA was administered intermittently if hemoglobin concentration decreased to less than 9 g/dL (15% versus 25%, respectively). In CHOIR, a randomized open-label study of 1432 patients with CKD not on dialysis, use of epoetin alfa to target a higher (13.5 g/dL) versus lower (11.3 g/dL) hemoglobin goal did not reduce the use of RBC transfusions. In each trial, no benefits occurred for the cardiovascular or end-stage renal disease outcomes. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile.
- ESA Effects on rates of death and other serious cardiac adverse events
- Three randomized outcome trials (Normal Hematocrit Study [NHS], Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease [CHOIR], and Trial of Darbepoetin Alfa in Type 2 Diabetes and CKD [TREAT]) have been conducted in patients with CKD using Epogen/PROCRIT/Aranesp to target higher vs. lower hemoglobin levels. Though these trials were designed to establish a cardiovascular or renal benefit of targeting higher hemoglobin levels, in all 3 studies, patients randomized to the higher hemoglobin target experienced worse cardiovascular outcomes and showed no reduction in progression to ESRD. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile.
Zidovudine-treated Patients With HIV Infection
- The safety and efficacy of Epogen were evaluated in 4 placebo-controlled studies enrolling 297 anemic patients (hemoglobin < 10 g/dL) with HIV infection receiving concomitant therapy with zidovudine. In the subgroup of patients (89/125 Epogen and 88/130 placebo) with pre study endogenous serum erythropoietin levels ≤ 500 mUnits/mL, Epogen reduced the mean cumulative number of units of blood transfused per patient by approximately 40% as compared to the placebo group. Among those patients who required RBC transfusions at baseline, 43% of patients treated with Epogen versus 18% of placebo-treated patients were RBC transfusion independent during the second and third months of therapy. Epogen therapy also resulted in significant increases in hemoglobin in comparison to placebo. When examining the results according to the weekly dose of zidovudine received during month 3 of therapy, there was a statistically significant reduction (p < 0.003) in RBC transfusion requirements in patients treated with Epogen (n = 51) compared to placebo-treated patients (n = 54) whose mean weekly zidovudine dose was ≤ 4200 mg/week.
- Approximately 17% of the patients with endogenous serum erythropoietin levels ≤ 500 mUnits/mL receiving Epogen in doses from 100 to 200 Units/kg 3 times weekly achieved a hemoglobin of 12.7 g/dL without administration of RBC transfusions or significant reduction in zidovudine dose. In the subgroup of patients whose pre study endogenous serum erythropoietin levels were > 500 mUnits/mL, Epogen therapy did not reduce RBC transfusion requirements or increase hemoglobin compared to the corresponding responses in placebo-treated patients.
Cancer Patients on Chemotherapy
- The safety and effectiveness of Epogen was assessed in two multicenter, randomized (1:1), placebo-controlled, double-blind studies (Study C1 and Study C2) and a pooled analysis of six additional randomized (1:1), multicenter, placebo-controlled, double-blind studies. All studies were conducted in patients with anemia due to concomitantly administered cancer chemotherapy. Study C1 enrolled 344 adult patients, Study C2 enrolled 222 pediatric patients, and the pooled analysis contained 131 patients randomized to epoetin alfa or placebo. In Studies C1 and C2, efficacy was demonstrated by a reduction in the proportion of patients who received an RBC transfusion, from week 5 through end of the study, with the last-known RBC transfusion status carried forward for patients who discontinued treatment. In the pooled analysis, efficacy was demonstrated by a reduction in the proportion of patients who received an RBC transfusion from week 5 through end of the study in the subset of patients who were remaining on therapy for 6 or more weeks.
- Study C1
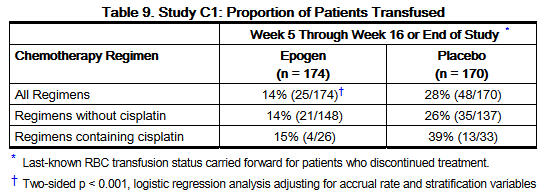
- Study C1 was conducted in anemic patients (hemoglobin < 11.5 g/dL for males; < 10.5 g/dL for females) with non-myeloid malignancies receiving myelosuppressive chemotherapy. Randomization was stratified by type of malignancy (lung vs. breast vs. other), concurrent radiation therapy planned (yes or no), and baseline hemoglobin (< 9 g/dL vs. ≥ 9 g/dL); patients were randomized to epoetin alfa 40,000 Units (n = 174) or placebo (n = 170) as a weekly subcutaneous injection commencing on the first day of the chemotherapy cycle.
- Ninety-one percent of patients were white, 44% were male, and the median age of patients was 66 years (range: 20 to 88 years). The proportion of patients withdrawn from the study prior to week 5 was less than 10% for placebo-treated or epoetin-treated patients. Per protocol, the last available hemoglobin values from patients who dropped out were included in the efficacy analyses. Efficacy results are shown in Table 9.
- Study C2
- Study C2 was conducted in 222 anemic patients, ages 5 to 18, receiving chemotherapy for the treatment of various childhood malignancies. Randomization was stratified by cancer type (solid tumors, Hodgkin’s disease, acute lymphocytic leukemia, vs. non-Hodgkin’s lymphoma); patients were randomized to receive epoetin alfa at 600 Units/kg maximum 40,000 Units (n = 111) or placebo (n = 111) as a weekly intravenous injection.
- Sixty-nine percent of patients were white, 55% were male, and the median age of patients was 12 years (range: 5 to 18 years). Two (2%) of placebo-treated patients and 3 (3%) of epoetin alfa-treated patients dropped out of the study prior to week 5. There were fewer RBC transfusions from week 5 through the end-of-study in epoetin-alfa treated patients [51% (57/111)] compared to placebo-treated patients [69% (77/111)]. There was no evidence of an improvement in health-related quality of life, including no evidence of an effect on fatigue, energy, or strength in patients receiving Epogen as compared to those receiving placebo.
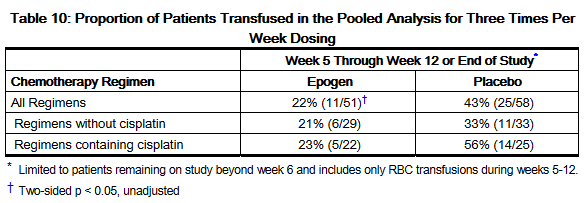
- Pooled Analysis (Three Times Per Week Dosing)
- The results of 6 studies of similar design and that randomized 131 patients to epoetin alfa or placebo were pooled to assess the safety and effectiveness of epoetin alfa. Patients were randomized to receive epoetin alfa at 150 Units/kg (n = 63) or placebo (n = 68), subcutaneously three times per week for 12 weeks in each study. Across all studies, 72 patients were treated with concomitant non cisplatin-containing chemotherapy regimens and 59 patients were treated with concomitant cisplatin-containing chemotherapy regimens. Twelve patients (19%) in the epoetin alfa arm and 10 patients (15%) in the placebo-arm dropped out prior to week 6 and are excluded from efficacy analyses.
Surgery Patients
- The safety and efficacy of Epogen were evaluated in a placebo-controlled, double-blind study (S1) enrolling 316 patients scheduled for major, elective orthopedic hip or knee surgery who were expected to require ≥ 2 units of blood and who were not able or willing to participate in an autologous blood donation program. Patients were stratified into 1 of 3 groups based on their pretreatment hemoglobin [≤ 10 g/dL (n = 2), > 10 to ≤ 13 g/dL (n = 96), and > 13 to ≤ 15 g/dL (n = 218)] and then randomly assigned to receive 300 Units/kg Epogen, 100 Units/kg Epogen, or placebo by subcutaneous injection for 10 days before surgery, on the day of surgery, and for 4 days after surgery. All patients received oral iron and a low-dose, postoperative warfarin regimen.
- Treatment with Epogen 300 Units/kg significantly (p = 0.024) reduced the risk of allogeneic RBC transfusion in patients with a pretreatment hemoglobin of > 10 to ≤ 13 g/dL; 5/31 (16%) of patients treated with Epogen 300 Units/kg, 6/26 (23%) of patients treated with Epogen 100 Units/kg, and 13/29 (45%) of placebo-treated patients were transfused. There was no significant difference in the number of patients transfused between Epogen (9% 300 Units/kg, 6% 100 Units/kg) and placebo (13%) in the > 13 to ≤ 15 g/dL hemoglobin stratum. There were too few patients in the ≤ 10 g/dL group to determine if Epogen is useful in this hemoglobin strata. In the > 10 to ≤ 13 g/dL pretreatment stratum, the mean number of units transfused per Epogen-treated patient (0.45 units blood for 300 Units/kg, 0.42 units blood for 100 Units/kg) was less than the mean transfused per placebo-treated patient (1.14 units) (overall p = 0.028). In addition, mean hemoglobin, hematocrit, and reticulocyte counts increased significantly during the presurgery period in patients treated with Epogen.
- Epogen was also evaluated in an open-label, parallel-group study (S2) enrolling 145 patients with a pretreatment hemoglobin level of ≥ 10 to ≤ 13 g/dL who were scheduled for major orthopedic hip or knee surgery and who were not participating in an autologous program. Patients were randomly assigned to receive 1 of 2 subcutaneous dosing regimens of Epogen (600 Units/kg once weekly for 3 weeks prior to surgery and on the day of surgery, or 300 Units/kg once daily for 10 days prior to surgery, on the day of surgery, and for 4 days after surgery). All patients received oral iron and appropriate pharmacologic anticoagulation therapy.
- From pretreatment to presurgery, the mean increase in hemoglobin in the 600 Units/kg weekly group (1.44 g/dL) was greater than that observed in the 300 Units/kg daily group. The mean increase in absolute reticulocyte count was smaller in the weekly group (0.11 × 106/mm3) compared to the daily group (0.17 × 106/mm3). Mean hemoglobin levels were similar for the 2 treatment groups throughout the postsurgical period.
- The erythropoietic response observed in both treatment groups resulted in similar RBC transfusion rates [11/69 (16%) in the 600 Units/kg weekly group and 14/71 (20%) in the 300 Units/kg daily group]. The mean number of units transfused per patient was approximately 0.3 units in both treatment groups.
How Supplied
- Store at 36ºF to 46ºF (2ºC to 8ºC). Do not freeze.
- Do not shake. Protect from light; store Epogen in the carton until use.
- Do not use Epogen that has been shaken or frozen.
- Single-dose, Preservative-free Vial (in citrate-buffered formulation): 1 mL of solution contains 2000 (NDC 55513-126-10), 3000 (NDC 55513-267-10), 4000 (NDC 55513-148-10), or 10,000 Units (NDC 55513-144-10) of epoetin alfa. Each strength is supplied in dispensing packs containing 10 single-dose vials.
- Single-dose, Preservative-free Vial (in phosphate-buffered formulation): 1 mL of solution contains 40,000 Units (NDC 55513-823-10) of epoetin alfa and is supplied in dispensing packs containing 10 single-dose vials.
- Multidose, Preserved Vial: 2 mL total volume (20,000 Units total; 10,000 Units/mL). Each 1 mL of solution contains 10,000 Units (NDC 55513-283-10) of epoetin alfa, and is supplied in dispensing packs containing 10 multidose vials.
- Multidose, Preserved Vial: 1 mL total volume (20,000 Units/mL). Each 1 mL of solution contains 20,000 Units (NDC 55513-478-10) of epoetin alfa and is supplied in dispensing packs containing 10 multidose vials.
Storage
There is limited information regarding Epoetin alfa Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Epoetin alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Epoetin alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Precautions with Alcohol
- Alcohol-Epoetin alfa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Epogen®[7]
Look-Alike Drug Names
There is limited information regarding Epoetin alfa Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E; et al. (2000). "The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations". J Am Coll Cardiol. 35 (7): 1737–44. PMID 10841219.
- ↑ Breymann C, Richter C, Hüttner C, Huch R, Huch A (2000). "Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anaemia and blunted erythropoiesis". Eur J Clin Invest. 30 (2): 154–61. PMID 10651841.
- ↑ Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS; et al. (2004). "Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study". Gastroenterology. 126 (5): 1302–11. PMID 15131791.
- ↑ Terpos E, Mougiou A, Kouraklis A, Chatzivassili A, Michalis E, Giannakoulas N; et al. (2002). "Prolonged administration of erythropoietin increases erythroid response rate in myelodysplastic syndromes: a phase II trial in 281 patients". Br J Haematol. 118 (1): 174–80. PMID 12100145.
- ↑ Cervantes F, Alvarez-Larrán A, Hernández-Boluda JC, Sureda A, Torrebadell M, Montserrat E (2004). "Erythropoietin treatment of the anaemia of myelofibrosis with myeloid metaplasia: results in 20 patients and review of the literature". Br J Haematol. 127 (4): 399–403. doi:10.1111/j.1365-2141.2004.05229.x. PMID 15521916.
- ↑ Chaidos A, Makis A, Hatzimichael E, Tsiara S, Gouva M, Tzouvara E; et al. (2004). "Treatment of beta-thalassemia patients with recombinant human erythropoietin: effect on transfusion requirements and soluble adhesion molecules". Acta Haematol. 111 (4): 189–95. doi:10.1159/000077551. PMID 15153710.
- ↑ "EPOGEN (epoetin alfa) solution [Amgen Inc]".
{{#subobject:
|Page Name=Epoetin alfa |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin12.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin13.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin14.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin15.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin16.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin17.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin18.png
}}
{{#subobject:
|Label Page=Epoetin alfa |Label Name=Epoetin19.png
}}