Emphysema pathophysiology
| https://https://www.youtube.com/watch?v=TEuSV_7gWA8%7C350}} |
|
Emphysema Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Emphysema pathophysiology On the Web |
|
American Roentgen Ray Society Images of Emphysema pathophysiology |
|
Risk calculators and risk factors for Emphysema pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
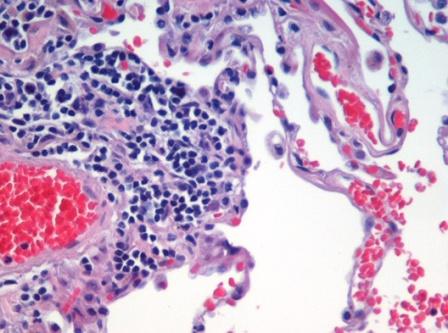
Emphysema is caused by loss of elasticity of the lung tissue, from destruction of structures supporting the alveoli, and destruction of capillaries feeding the alveoli. The result is that the small airways collapse during exhalation (although alveolar collapsability has increased), leading to an obstructive form of lung disease (airflow is impeded and air is generally "trapped" in the lungs in obstructive lung diseases). When toxins such as smoke are breathed into the lungs, the particles are trapped and cause a localized inflammatory response. Chemicals released during the inflammatory response (e.g., elastase) can break down the walls of alveoli (alveolar septum). This leads to fewer but larger alveoli, with a decreased surface area and a decreased ability to absorb oxygen and exude carbon dioxide by diffusion. The activity of another molecule called alpha 1-antitrypsin normally neutralizes the destructive action of one of these damaging molecules.
Pathophysiology
Emphysema is caused by loss of elasticity (increased compliance) of the lung tissue, from destruction of structures supporting the alveoli, and destruction of capillaries feeding the alveoli. The result is that the small airways collapse during exhalation (although alveolar collapsability has increased), leading to an obstructive form of lung disease (airflow is impeded and air is generally "trapped" in the lungs in obstructive lung diseases).
- When toxins such as smoke are breathed into the lungs, the particles are trapped and cause a localized inflammatory response. Chemicals released during the inflammatory response (e.g., elastase) can break down the walls of alveoli (alveolar septum). This leads to fewer but larger alveoli, with a decreased surface area and a decreased ability to absorb oxygen and exude carbon dioxide by diffusion. The activity of another molecule called alpha 1-antitrypsin normally neutralizes the destructive action of one of these damaging molecules.
- After a prolonged period, hyperventilation becomes inadequate to maintain high enough oxygen levels in the blood. The body compensates by vasoconstricting appropriate vessels. This leads to pulmonary hypertension, which places increased strain on the right side of the heart, the one that pumps unoxygenated blood to the lungs, fails. The failure causes the heart muscle to thicken to pump more blood. Eventually, as the heart continues to fail, it becomes larger and blood backs up in the liver.
- Emphysema occurs in a higher proportion in patients with decreased alpha 1-antitrypsin (A1AT) levels (alpha 1-antitrypsin deficiency, A1AD). In A1AD, inflammatory enzymes (such as elastase) are able to destroy the alveolar tissue (the elastin fibre, for example). Most A1AD patients do not develop clinically significant emphysema, but smoking and severely decreased A1AT levels (10-15%) can cause emphysema at a young age. In all, A1AD causes about 2% of all emphysema. However, smokers with A1AD are in the highest risk category for emphysema.
- While A1AD provides some insight into the pathogenesis of the disease, hereditary A1AT deficiency only accounts for a small proportion of the disease. Studies for the better part of the past century have focused mainly upon the putative role of leukocyte elastase (also neutrophil elastase), a serine protease found in neutrophils, as a primary contributor to the connective tissue damage seen in the disease. This hypothesis, a result of the observation that NE is the primary substrate for A1AT, and A1AT is the primary inhibitor of NE, together have been known as the "protease-antiprotease" theory, implicating neutrophils as an important mediator of the disease. However, more recent studies have brought into light the possibility that one of the many other numerous proteases, especially matrix metalloproteases might be equally or more relevant than NE in the development of non-hereditary emphysema.
- The better part of the past few decades of research into the pathogenesis of emphysema involved animal experiments where various proteases were instilled into the trachea of various species of animals. These animals developed connective tissue damage, which was taken as support for the protease-antiprotease theory. However, just because these substances can destroy connective tissue in the lung, as anyone would be able to predict, doesn't establish causality. More recent experiments have focused on more technologically advanced approaches, such as ones involving genetic manipulation. Perhaps the most interesting development with respect to our understanding of the disease involves the production of protease "knock-out" animals, which are genetically deficient in one or more proteases, and the assessment of whether they would be less susceptible to the development of the disease
Gross Pathology

Microscopic Pathology