Dyphylline
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dyphylline is a bronchodilator that is FDA approved for the treatment of acute bronchial asthma and for reversible bronchospasm associated with chronic bronchitis and emphysema. Common adverse reactions include diarrhea, nausea, vomiting, headache, agitation, feeling excited, irritability.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- For relief of acute bronchial asthma and for reversible bronchospasm associated with chronic bronchitis and emphysema.
Dosage
- Dosage should be individually titrated according to the severity of the condition and the response of the patient.
- Usual adult dosage: Up to 15 mg/kg every six hours.
- Appropriate dosage adjustments should be made in patients with impaired renal function
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Dyphylline in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Dyphylline in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Dyphylline in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Dyphylline in pediatric patients.
Contraindications
- Hypersensitivity to dyphylline or related xanthine compounds.
Warnings
- Dyphylline is not indicated in the management of status asthmaticus, which is a serious medical emergency.
- Although the relationship between plasma levels of dyphylline and appearance of toxicity is unknown, excessive doses may be expected to be associated with an increased risk of adverse effects.
Precautions
- General: Use dyphylline with caution in patients with severe cardiac disease, hypertension, hyperthyroidism, acute myocardial injury, or peptic ulcer.
- Concurrent administration of dyphylline and probenecid, which competes for tubular secretion, has been shown to increase the plasma half-life of dyphylline.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions with the use of dyphylline have been infrequent, relatively mild, and rarely required reduction in dosage or withdrawal of therapy.
- The following adverse reactions which have been reported with other xanthine bronchodilators, and which have most often been related to excessive drug plasma levels, should be considered as potential adverse effects when dyphylline is administered:
- Gastrointestinal: nausea, vomiting, epigastric pain, hematemesis, diarrhea.
- Central nervous system: headache, irritability, restlessness, insomnia, hyperexcitability, agitation, muscle twitching, generalized clonic and tonic convulsions.
- Cardiovascular: palpitation, tachycardia, extrasystoles, flushing, hypotension, circulatory failure, ventricular arrhythmias.
- Respiratory: tachypnea.
- Renal: albuminuria, gross and microscopic hematuria, diuresis.
- Other: hyperglycemia, inappropriate ADH syndrome.
Postmarketing Experience
- There is limited information regarding Postmarketing experience of Dyphylline.
Drug Interactions
- Synergism between xanthine bronchodilators (e.g., theophylline), ephedrine, and other sympathomimetic bronchodilators has been reported. This should be considered whenever these agents are prescribed concomitantly.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with dyphylline. It is also not known if dyphylline can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dyphylline should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dyphylline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dyphylline during labor and delivery.
Nursing Mothers
- Dyphylline is present in human milk at approximately twice the maternal plasma concentration. Caution should be exercised when dyphylline is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
- Clinical studies of dyphylline did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Dyphylline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dyphylline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dyphylline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dyphylline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dyphylline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dyphylline in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Monitoring and maintenance of vital signs, fluids, and electrolytes in overdosage.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- There have been no reports, in the literature, of overdosage with dyphylline. However, the following information based on reports of theophylline overdosage are considered typical of the xanthine class of drugs and should be kept in mind.
- Signs and symptoms: Restlessness, anorexia, nausea, vomiting, diarrhea, insomnia, irritability, and headache. Marked overdosage with resulting severe toxicity has produced agitation, severe vomiting, dehydration, excessive thirst, tinnitus, cardiac arrhythmias, hyperthermia, diaphoresis, and generalized clonic and tonic convulsions. Cardiovascular collapse has also occurred, with some fatalities. Seizures have occurred in some cases associated with very high theophylline plasma concentrations, without any premonitory symptoms of toxicity.
- Treatment: There is no specific antidote for overdosage with drugs of the xanthine class. Symptomatic treatment and general supportive measures should be instituted with careful monitoring and maintenance of vital signs, fluids, and electrolytes. The stomach should be emptied by inducing emesis if the patient is conscious and responsive, or by gastric lavage, taking care to protect against aspiration, especially in stuporous or comatose patients. Maintenance of an adequate airway is essential in case oxygen or assisted respiration is needed. Sympathomimetic agents should be avoided but sedatives such as short-acting barbiturates may be useful.
- Dyphylline is dialyzable and, although not recommended as a routine procedure in overdosage cases, hemodialysis may be of some benefit when severe intoxication is present or when the patient has not responded to general supportive and symptomatic treatment.
Pharmacology
| |
Dyphylline
| |
| Systematic (IUPAC) name | |
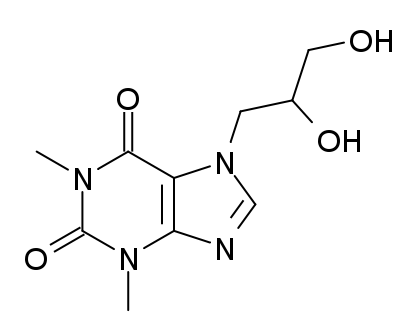
| 7-(2,3-dihydroxypropyl)-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione | |
| Identifiers | |
| CAS number | |
| ATC code | R03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 254.24 g/mol |
| SMILES | & |
| Synonyms | 7-(2,3-dihydroxy-propyl)theophylline |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
Dyphylline is a xanthine derivative with pharmacologic actions similar to theophylline and other members of this class of drugs. Its primary action is that of bronchodilation, but it also exhibits peripheral vasodilatory and other smooth muscle relaxant activity to a lesser degree. The bronchodilatory action of dyphylline, as with other xanthines, is thought to be mediated through competitive inhibition of phosphodiesterase with a resulting increase in cyclic AMP producing relaxation of bronchial smooth muscle.
Structure
Dyphylline, a xanthine derivative, is a bronchodilator available for oral administration as tablets containing 200 mg and 400 mg of dyphylline. Other ingredients: magnesium stearate, microcrystalline cellulose.
Chemically, dyphylline is 7-(2,3-dihydroxypropyl)-theophylline, a white, extremely bitter, amorphous powder that is freely soluble in water and soluble in alcohol to the extent of 2 g/100 mL. Dyphylline forms a neutral solution that is stable in gastrointestinal fluids over a wide range of pH.
The molecular formula for dyphylline is C10H14N4O4 with a molecular weight of 254.25. Its structural formula is:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dyphylline.
Pharmacokinetics
- Dyphylline is well tolerated and produces less nausea than aminophylline and other alkaline theophylline compounds when administered orally. Unlike the hydrolyzable salts of theophylline, dyphylline is not converted to free theophylline in vivo. It is absorbed rapidly in therapeutically active form and in healthy volunteers reaches a mean peak plasma concentration of 17.1 mcg/mL in approximately 45 minutes following a single oral dose of 1000 mg of dyphylline.
- Dyphylline exerts its bronchodilatory effects directly and, unlike theophylline, is excreted unchanged by the kidneys without being metabolized by the liver. Because of this, dyphylline pharmacokinetics and plasma levels are not influenced by various factors that affect liver function and hepatic enzyme activity, such as smoking, age, congestive heart failure, or concomitant use of drugs which affect liver function.
- The elimination half-life of dyphylline is approximately two hours (1.8-2.1 hr) and approximately 88% of a single oral dose can be recovered from the urine unchanged. The renal clearance would be correspondingly reduced in patients with impaired renal function. In anuric patients, the half-life may be increased 3 to 4 times normal.
- Dyphylline plasma levels are dose-related and generally predictable. The range of plasma levels within which dyphylline can be expected to produce effective bronchodilation has not been determined.
Nonclinical Toxicology
- Carcinogenesis, mutagenesis, impairment of fertility: No long-term animal studies have been performed with dyphylline.
Clinical Studies
- There is limited information regarding Clinical Studies of Dyphylline.
How Supplied
- LUFYLLIN Tablets contain 200 mg dyphylline and are white, rectangular, scored on one side and imprinted WALLACE 521 on the other side. The tablets are available in bottles of 100 (NDC 0037-0521-92).
- LUFYLLIN-400 Tablets contain 400 mg dyphylline and are white, capsule-shaped, scored on one side and imprinted WALLACE 731 on the other side. The tablets are available in bottles of 100 (NDC 0037-0731-92).
Storage
- Store at controlled room temperature 20°-25°C (68°-77°F).
- Dispense in a tight container.
Images
Drug Images
{{#ask: Page Name::Dyphylline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Dyphylline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dyphylline Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Dyphylline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LUFYLLIN ®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.