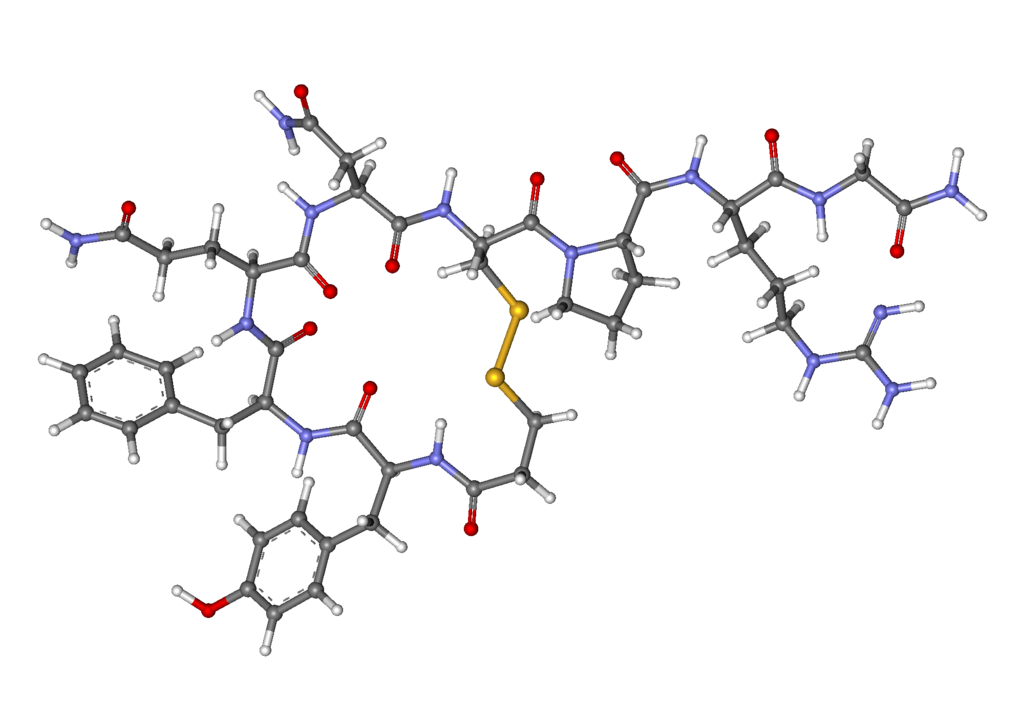
Desmopressin acetate nasal spray
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Desmopressin acetate nasal spray is a hormone analog that is FDA approved for the treatment of as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. Common adverse reactions include fatigue, rhinitis, myocardial infarction, hyponatremia, hyposmolality, water intoxication syndrome, anaphylaxis, seizure.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Central Cranial Diabetes Insipidus
- DDAVP Nasal Spray is indicated as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. It is ineffective for the treatment of nephrogenic diabetes insipidus.
- The use of DDAVP Nasal Spray in patients with an established diagnosis will result in a reduction in urinary output with increase in urine osmolality and a decrease in plasma osmolality. This will allow the resumption of a more normal life-style with a decrease in urinary frequency and nocturia.
- There are reports of an occasional change in response with time, usually greater than 6 months. Some patients may show a decreased responsiveness, others a shortened duration of effect. There is no evidence this effect is due to the development of binding antibodies but may be due to a local inactivation of the peptide.
- Patients are selected for therapy by establishing the diagnosis by means of the water deprivation test, the hypertonic saline infusion test, and/or the response to antidiuretic hormone. Continued response to intranasal DDAVP can be monitored by urine volume and osmolality.
- DDAVP is also available as a solution for injection when the intranasal route may be compromised. These situations include nasal congestion and blockage, nasal discharge, atrophy of nasal mucosa, and severe atrophic rhinitis. Intranasal delivery may also be inappropriate where there is an impaired level of consciousness. In addition, cranial surgical procedures, such as transsphenoidal hypophysectomy create situations where an alternative route of administration is needed as in cases of nasal packing or recovery from surgery.
- DDAVP Nasal Spray dosage must be determined for each individual patient and adjusted according to the diurnal pattern of response. Response should be estimated by two parameters: adequate duration of sleep and adequate, not excessive, water turnover. Patients with nasal congestion and blockage have often responded well to intranasal DDAVP.
- The usual dosage range in adults is 0.1 to 0.4 mL daily, either as a single dose or divided into two or three doses. Most adults require 0.2 mL daily in two divided doses.
- The morning and evening doses should be separately adjusted for an adequate diurnal rhythm of water turnover. For children aged 3 months to 12 years, the usual dosage range is 0.05 to 0.3 mL daily, either as a single dose or divided into two doses. About 1/4 to 1/3 of patients can be controlled by a single daily dose of DDAVP administered intranasally. Fluid restriction should be observed.
- The nasal spray pump can only deliver doses of 0.1 mL (10 mcg) or multiples of 0.1 mL. If doses other than these are required, the rhinal tube delivery system may be used.
- The spray pump must be primed prior to the first use. To prime pump, press down four times. The bottle will now deliver 10 mcg of drug per spray.
- Discard DDAVP Nasal Spray after 50 sprays since the amount delivered thereafter per spray may be substantially less than 10 µg of drug.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Vasopressin equivalent antidiuretic activity
- (Solution for injection) 4 mcg is equivalent to 16 international units of vasopressin; (DDAVP(R) nasal spray) 0.1 mL (10 mcg) per spray equivalent to 40 international units; (rhinal tube) 1 mL (0.1 mg) equivalent to 400 international units; (Stimate(R) nasal spray) 0.1 mL (150 mcg) per spray equivalent to 600 international units
- Hemophilia A, With factor VIII levels greater than 5%: 0.3 mcg/kg diluted in 50 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more often than every 48 hours
- Hemophilia A, With factor VIII levels greater than 5%: (Stimate(R)) less than 50 kg, 1 spray (150 mcg) INTRANASALLY in 1 nostril only, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response
- Hemophilia A, With factor VIII levels greater than 5%: (Stimate(R)) 50 kg and over, 1 spray (150 mcg) INTRANASALLY in each nostril, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response
- Neurohypophyseal diabetes insipidus: (DDAVP(R)) 10 to 40 mcg/day (0.1 mL to 0.4 mL) INTRANASALLY, either as a single dose or 2 to 3 divided doses
- Neurohypophyseal diabetes insipidus
- 2 to 4 mcg/day IV or SUBQ in 2 divided doses; comparable antidiuretic dose of the injection is about one-tenth (1/10) the intranasal dose
- Neurohypophyseal diabetes insipidus
- initial, 0.05 mg (one-half of the 0.1-mg tablet) ORALLY twice daily; maintenance 0.1 to 0.8 mg/day in divided doses
- (Men) Orally disintegrating tablet, 100 mcg ORALLY once daily 1 hour before bedtime
- (Women) Orally disintegrating tablet, 25 to 100 mcg ORALLY once daily 1 hour before bedtime[1]
- Primary nocturnal enuresis
- Initial, 0.2 mg ORALLY at bedtime; dose may be titrated up to 0.6 mg if necessary
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: 0.3 mcg/kg diluted in 50 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more often than every 48 hours
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: (Stimate(R)) less than 50 kg, 1 spray (150 mcg) INTRANASALLY in one nostril only, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: (Stimate(R)) 50 kg and over, 1 spray (150 mcg) INTRANASALLY in each nostril, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response
Non–Guideline-Supported Use
- Nocturia
- Urinary incontinence
- Urine concentration test
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Central Cranial Diabetes Insipidus
- DDAVP Nasal Spray has been used in children with diabetes insipidus. Use in infants and children will require careful fluid intake restriction to prevent possible hyponatremia and water intoxication.
- The dose must be individually adjusted to the patient with attention in the very young to the danger of an extreme decrease in plasma osmolality with resulting convulsions. Dose should start at 0.05 mL or less.
- Since the spray cannot deliver less than 0.1 mL (10 mcg), smaller doses should be administered using the rhinal tube delivery system. Do not use the nasal spray in pediatric patients requiring less than 0.1 mL (10 mcg) per dose.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- Nasal spray formulations should not be used in pediatric patients requiring less than 0.1 mL or 10 mcg per dose.
- Vasopressin equivalent antidiuretic activity
- (Solution for injection) 4 mcg is equivalent to 16 international units of vasopressin; (DDAVP(R) nasal spray) 0.1 mL (10 mcg) per spray equivalent to 40 international units; (rhinal tube) 1 mL (0.1 mg) equivalent to 400 international units; (Stimate(R) nasal spray) 0.1 mL (150 mcg) per spray equivalent to 600 international units
- Hemophilia A, With factor VIII levels greater than 5%
- 3 months or older (10 kg or less), 0.3 mcg/kg diluted in 10 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more often than every 48 hours
- Hemophilia A, With factor VIII levels greater than 5%: 3 months or older (more than 10 kg), 0.3 mcg/kg diluted in 50 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more than every 48 hours
- Hemophilia A, With factor VIII levels greater than 5%
- (Stimate(R)) 11 months or older (less than 50 kg), 1 spray (150 mcg) INTRANASALLY in one nostril only, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response.
- Hemophilia A, With factor VIII levels greater than 5%
- (Stimate(R)) 11 months or older (50 kg or over), 1 spray (150 mcg) INTRANASALLY in each nostril, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response
- Neurohypophyseal diabetes insipidus
- (DDAVP(R)) 3 months to 12 years, 5 to 30 mcg (0.05 mL to 0.3 mL) INTRANASALLY, either as a single dose or 2 to 3 divided doses; for doses less than 10 mcg use the rhinal tube system.
- Neurohypophyseal diabetes insipidus
- (DDAVP(R)) 13 years or older, 10 to 40 mcg/day (0.1 mL to 0.4 mL) INTRANASALLY, either as a single dose or 2 to 3 divided doses.
- Neurohypophyseal diabetes insipidus
- 12 years or older, 2 to 4 mcg/day IV or SUBQ in 2 divided doses; comparable antidiuretic dose of the injection is about one-tenth (1/10) the intranasal dose.
- Neurohypophyseal diabetes insipidus
- 4 years or older, initial, 0.05 mg (one-half of the 0.1-mg tablet) ORALLY twice daily; maintenance, 0.1 to 0.8 mg/day in divided doses.
- Primary nocturnal enuresis
- 6 years or older: initial, 0.2 mg ORALLY at bedtime, dose may be titrated up to 0.6 mg if necessary.
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: 3 months or older (10 kg or less), 0.3 mcg/kg diluted in 10 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more often than every 48 hours.
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: 3 months or older (more than 10 kg), 0.3 mcg/kg diluted in 50 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses; tachyphylaxis may occur if given more than every 48 hours.
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: (Stimate(R)) 11 months or older (less than 50 kg), 1 spray (150 mcg) INTRANASALLY in 1 nostril only, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response.
- von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%: (Stimate(R)) 11 months or older (50 kg or over), 1 spray (150 mcg) INTRANASALLY in each nostril, may be repeated based on laboratory response and clinical condition; perform test dose prior to therapeutic use to confirm appropriate coagulation profile response.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desmopressin acetate in pediatric patients.
Contraindications
- DDAVP Nasal Spray is contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of DDAVP Nasal Spray.
- DDAVP is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50ml/min).
- DDAVP is contraindicated in patients with hyponatremia or a history of hyponatremia.
Warnings
For intranasal use only
- DDAVP Nasal Spray should only be used in patients where orally administered formulations are not feasible.
- Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with DDAVP (desmopressin acetate). DDAVP is a potent antidiuretic which, when administered, may lead to water intoxication and/or hyponatremia.
- Unless properly diagnosed and treated hyponatremia can be fatal. Therefore, fluid restriction is recommended and should be discussed with the patient and/or guardian. Careful medical supervision is required.
- When DDAVP Nasal Spray is administered, in particular in pediatric and geriatric patients, fluid intake should be adjusted downward in order to decrease the potential occurrence of water intoxication and hyponatremia.
- All patients receiving DDAVP therapy should be observed for the following signs or symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss of appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness and confusion.
- Severe symptoms may include one or a combination of the following: seizure, coma and/or respiratory arrest. Particular attention should be paid to the possibility of the rare occurrence of an extreme decrease in plasma osmolality that may result in seizures which could lead to coma.
- DDAVP should be used with caution in patients with habitual or psychogenic polydipsia who may be more likely to drink excessive amounts of water, putting them at greater risk of hyponatremia.
Adverse Reactions
Clinical Trials Experience
Infrequently, high dosages of intranasal DDAVP have produced transient headache and nausea. Nasal congestion, rhinitis and flushing have also been reported occasionally along with mild abdominal cramps. These symptoms disappeared with reduction in dosage. Nosebleed, sore throat, cough and upper respiratory infections have also been reported.
The following table lists the percentage of patients having adverse experiences without regard to relationship to study drug from the pooled pivotal study data for nocturnal enuresis.

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Postmarketing Experience
- There have been rare reports of hyponatremic convulsions associated with concomitant use with the following medications: oxybutinin and imipramine.
Drug Interactions
There is limited information regarding Desmopressin acetate nasal spray Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Fertility studies have not been done. Teratology studies in rats and rabbits at doses from 0.05 to 10 mcg/kg/day (approximately 0.1 times the maximum systemic human exposure in rats and up to 38 times the maximum systemic human exposure in rabbits based on surface area, mg/m2) revealed no harm to the fetus due to DDAVP (desmopressin acetate).
- There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Several publications of desmopressin acetate's use in the management of diabetes insipidus during pregnancy are available; these include a few anecdotal reports of congenital anomalies and low birth weight babies. However, no causal connection between these events and desmopressin acetate has been established.
- A fifteen year Swedish epidemiologic study of the use of desmopressin acetate in pregnant women with diabetes insipidus found the rate of birth defects to be no greater than that in the general population; however, the statistical power of this study is low.
- As opposed to preparations containing natural hormones, desmopressin acetate in antidiuretic doses has no uterotonic action and the physician will have to weigh the therapeutic advantages against the possible risks in each case.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desmopressin acetate nasal spray in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desmopressin acetate nasal spray during labor and delivery.
Nursing Mothers
- There have been no controlled studies in nursing mothers. A single study in a post-partum woman demonstrated a marked change in plasma, but little if any change in assayable DDAVP in breast milk following an intranasal dose of 10 mcg.
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DDAVP is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Desmopressin acetate nasal spray in pediatric settings.
Geriatic Use
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Desmopressin acetate nasal spray with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desmopressin acetate nasal spray with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Desmopressin acetate nasal spray in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desmopressin acetate nasal spray in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desmopressin acetate nasal spray in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desmopressin acetate nasal spray in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Although the pressor activity of DDAVP is very low compared to the antidiuretic activity, use of large doses of intranasal DDAVP with other pressor agents should only be done with careful patient monitoring.
IV Compatibility
There is limited information regarding the compatibility of Desmopressin acetate nasal spray and IV administrations.
Overdosage
- Signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention.
- In case of overdosage, the dose should be reduced, frequency of administration decreased, or the drug withdrawn according to the severity of the condition. There is no known specific antidote for desmopressin acetate or DDAVP Nasal Spray.
- An oral LD50 has not been established. An intravenous dose of 2 mg/kg in mice demonstrated no effect.
Pharmacology
Mechanism of Action
- Desmopressin works by limiting the amount of water that is eliminated in the urine. It works at the level of the renal collecting duct by binding to V2 receptors, which signal for the translocation of aquaporin channels via cytosolic vesicles to the apical membrane of the collecting duct.
- The presence of these aquaporin channels in the distal nephron causes increasing water reabsorption from the urine, which becomes passively re-distributed from the nephron to systemic circulation by way of basolateral membrane channels.[2]
- Desmopressin also stimulates release of von Willebrand factor from endothelial cells by acting on the V2 receptor.
Desmopressin is degraded more slowly than recombinant vasopressin, and requires less frequent administration. In addition, it has little effect on blood pressure, while vasopressin may cause arterial hypertension. Vasopressin stimulates the release of ACTH, which indirectly increases responsiveness of alpha-1 receptor in blood vessel smooth muscle, increasing vessel tone and blood pressure.
Structure
- DDAVP® Nasal Spray (desmopressin acetate) is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
- Mol. wt. 1183.34
- Empirical formula: C46H64N14O12S2•C2H4O2•3H2O
Pharmacodynamics
- DDAVP contains as active substance desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin. One mL (0.1 mg) of intranasal DDAVP has an antidiuretic activity of about 400 IU; 10 mcg of desmopressin acetate is equivalent to 40 IU.
- The biphasic half-lives for intranasal DDAVP were 7.8 and 75.5 minutes for the fast and slow phases, compared with 2.5 and 14.5 minutes for lysine vasopressin, another form of the hormone used in this condition. As a result, intranasal DDAVP provides a prompt onset of antidiuretic action with a long duration after each administration.
- The change in structure of arginine vasopressin to DDAVP has resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses are usually below threshold levels for effects on vascular or visceral smooth muscle.
- DDAVP administered intranasally has an antidiuretic effect about one-tenth that of an equivalent dose administered by injection.
Pharmacokinetics
- DDAVP is mainly excreted in the urine. A pharmacokinetic study conducted in healthy volunteers and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) receiving single dose desmopressin acetate (2mcg) injection demonstrated a difference in DDAVP terminal half-life.
- Terminal half-life significantly increased from 3 hours in normal healthy patients to 9 hours in patients with severe renal impairment.
Nonclinical Toxicology
There is limited information regarding Desmopressin acetate nasal spray Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Desmopressin acetate nasal spray Clinical Studies in the drug label.
How Supplied
- DDAVP Nasal Spray is available in a 5-mL bottle with spray pump delivering 50 sprays of 10 mcg (NDC 0075-2452-01). Desmopressin acetate is also available as DDAVP Rhinal Tube, a refrigerated product with 2.5 mL per bottle, packaged with two rhinal tube applicators per carton (NDC 0075-2450-01).
Storage
- Store at Controlled Room Temperature 20 to 25°C (68 to 77°F) [see USP]. STORE BOTTLE IN UPRIGHT POSITION.
- Keep out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Desmopressin acetate nasal spray |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desmopressin acetate nasal spray |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- A better way to deliver DDAVP
- Delivering DDAVP more efficiently
- Your doctor has prescribed DDAVP as antidiuretic hormone replacement therapy. Follow the dosage schedule that is specified. * The convenient nasal spray pump provides an efficient, reliable way to administer your medication. It is important, however, to adhere completely to the following instructions so that you will always receive a consistent dose of your medication.
- CAUTION: The nasal spray pump accurately delivers 50 doses of 10 micrograms each. Any solution remaining after 50 doses should be discarded since the amount delivered thereafter per actuation may be substantially less than 10 micrograms of drug. Do not transfer any remaining solution to another bottle. Please read the following instructions carefully before using the spray pump.
- Ensure that in children administration is under adult supervision in order to control the dose intake.
- If you accidentally deliver/administer too much of a dose, immediately telephone your doctor or a certified Regional Poison Center for advice. Possible signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention.
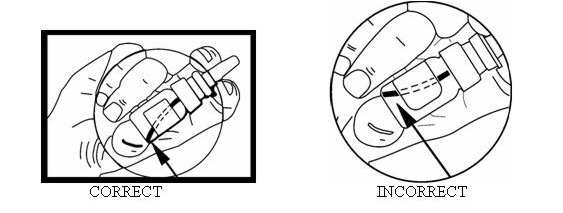
- Using your DDAVP Nasal Spray Pump
- Remove protective cap.
- The spray pump must be primed prior to the first use. To prime pump, press down 4 times.
- Once primed, the spray pump delivers 10 micrograms of medication each time it is pressed. To ensure dosing accuracy, tilt bottle so that dip tube inside the bottle draws from the deepest portion of the medication.
- To administer a 10-microgram dose, place the spray nozzle in nostril and press the spray pump once. If a higher dose has been prescribed, spray half the dose in each nostril. The spray pump cannot be used for doses less than 10 micrograms or doses other than multiples of 10 micrograms.
- Replace the protective cap on bottle after use. The pump will stay primed for up to one week. If the product has not been used for a period of one week, re-prime the pump by pressing once.
We have included a convenient check-off chart to assist you in keeping track of medication doses used. This will help assure that you receive 50 "full doses" of medication. Please note that the bottle has been filled with extra solution to accommodate the initial priming activity.
- Retain with medication or affix in convenient location.
Starting with dose #1, check off after each administration. Discard medication after 50 doses.
Precautions with Alcohol
Alcohol-Desmopressin acetate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DDAVP®[3]
Look-Alike Drug Names
There is limited information regarding Desmopressin acetate nasal spray Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Weiss JP, Zinner NR, Klein BM, Nørgaard JP (2012). "Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trial". Neurourol Urodyn. 31 (4): 441–7. doi:10.1002/nau.22243. PMID 22447415 PMID: 22447415 Check
|pmid=value (help). - ↑ Friedman FM, Weiss JP (2013). "Desmopressin in the treatment of nocturia: clinical evidence and experience". Ther Adv Urol. 5 (6): 310–7. doi:10.1177/1756287213502116. PMC 3825109. PMID 24294289 PMID 24294289 Check
|pmid=value (help). - ↑ "DDAVP- desmopressin acetate solution".
{{#subobject:
|Label Page=Desmopressin acetate nasal spray |Label Name=DDAP 6.jpg
}}
{{#subobject:
|Label Page=Desmopressin acetate nasal spray |Label Name=DDAP 7.jpg
}}
{{#subobject:
|Label Page=Desmopressin acetate nasal spray |Label Name=DailyMed - DDAVP- desmopressin acetate solution .png
}}
|drugShortage= }}