Daunorubicin liposome
For patient information regarding Daunorubicin liposome, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Steven Bellm, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.Black Box Warning
|
WARNINGS
See full prescribing information for complete Boxed Warning.
* Cardiac function should be monitored regularly in patients receiving Daunoxome (daunorubicin citrate liposome injection) because of the potential risk for cardiac toxicity and congestive heart failure. Cardiac monitoring is advised especially in those patients who have received prior anthracyclines or who have pre-existing cardiac disease or who have had prior radiotherapy encompassing the heart.
|
Overview
Daunorubicin liposome is an anthracycline that is FDA approved for the treatment of advanced HIV-associated Kaposi's sarcoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cardiotoxicity and myelosuppression.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Colon cancer
In a phase II clinical trial, Daunorubicin citrate liposome in doses of 100 milligrams/square meter every 3 weeks was not associated with significant antitumor activity in patients with metastatic Adenocarcinoma of the colon progressing after 5-fluorouracil Do not mix Daunoxome with other drugs.
Kaposi's sarcoma, First line therapy; in patients with advanced HIV-related Kaposi's sarcoma
- Important note: The dose OF Daunorubicin citrate liposome is different than that of conventional Daunorubicin and the two formulations are not interchangeable. Consult the drug evaluation monograph entitled "daunorubicin" for clinical use of this formulation.
- Daunorubicin citrate liposome is indicated as a first-line cytotoxic therapy for advanced HIV-associated Kaposi's sarcoma. It is not recommended in patients with less than advanced Kaposi's sarcoma. The dose of Daunorubicin citrate liposome is 40 milligrams/square meter, administered intravenously over 60 minutes, every 2 weeks. Blood counts should be repeated prior to each dose, and therapy withheld if the absolute granulocyte count is less than 750 cells/cubic millimeter. Treatment should be continued until there is evidence of progressive disease (eg, based on best response achieved: new visceral sites of involvement, or progression of visceral disease; development of 10 or more new cutaneous lesions or a 25% increase in the number of lesions compared to baseline; a change in the character of 25% or more of all previously counted flat lesions to raised; increase in the surface area the indicator lesions), or until other complications of HIV disease require discontinuation of therapy.
- In the treatment of AIDS-related Kaposi's sarcoma, the recommended dose of Daunorubicin citrate liposome is 40 milligrams/square meter every 14 days. The median duration of response in one study was 12 weeks.
- Important Note: The dose of Daunorubicin citrate liposome is different than that of conventional Daunorubicin and the two formulations are not interchangeable. Consult the Drug Evaluation monograph entitled "Daunorubicin" for clinical use of this formulation.
- Infiltration: Care should be given to avoid extravasation.
- Intravenous rate of administration: Daunorubicin citrate liposome should be administered diluted in 5% Dextrose in Water for Injection in volumes of 100 mL and administered via intravenous infusion over 60 minutes. The manufacturer of Daunorubicin citrate liposome emphasizes that an inline filter should not be used.
- Intravenous solution preparation
- Daunorubicin citrate liposome should be diluted 1:1 with 5% Dextrose Injection (D5W) before administration. Each vial of Daunoxome(R) contains Daunorubicin citrate equivalent to 50 milligrams Daunorubicin base, at a concentration of 2 mg/mL. The recommended concentration after dilution is 1 mg/mL. The only fluid which may be mixed with Daunoxome(R) is 5% Dextrose Injection; the drug must not be mixed with saline, bacteriostatic agents such as benzyl alcohol, or any other solution. Strictly observe aseptic technique in all handling. Do not use an inline filter for intravenous infusion of DaunoXome.
- The calculated volume of Daunorubicin citrate liposome should be drawn from the vial into a sterile syringe, and transferred into a sterile infusion bag containing an equivalent volume of 5% Dextrose Injection (D5W). Diluted Daunorubicin citrate liposome should be administered immediately; if not used immediately, the dose should be stored refrigerated at 2 to 8 degrees Centigrade (36 to 46 degrees Fahrenheit) for a maximum of 6 hours.
Dosage in Renal Failure
There is limited clinical experience in treating patients with renal impairment with Daunorubicin citrate liposome. Thus, based on experience with conventional Daunorubicin hydrochloride, it is recommended that the dose of Daunorubicin citrate liposome be reduced if the serum creatinine is elevated as follows: For serum creatinine greater than 3 mg/dL, give one-half the normal dose.
Dosage in Hepatic Insufficiency
There is limited clinical experience in treating patients with hepatic impairment with Daunorubicin citrate liposome. Thus, based on experience with Daunorubicin hydrochloride, it is recommended that the dose of Daunorubicin citrate liposome be reduced if the serum bilirubin is elevated as follows: For serum bilirubin 1.2 to 3 milligrams/deciliter, give three-fourths the normal dose; for serum bilirubin greater than 3 mg/dL, give one-half the normal dose.
Geriatric Use
The safety and efficacy of Daunorubicin citrate liposome in the elderly have not been established
There is limited information regarding Off-Label Guideline-Supported Use of Daunorubicin liposome in adult patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Daunorubicin liposome in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Daunorubicin liposome in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Daunorubicin liposome in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Daunorubicin liposome in pediatric patients.
Contraindications
Therapy with Daunoxome is contraindicated in patients who have experienced a serious hypersensitivity reaction to previous doses of Daunoxome or to any of its constituents.
Warnings
Daunoxome is intended for administration under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents.
The primary toxicity of Daunoxome is myelosuppression, especially of the granulocytic series, which may be severe, and associated with fever and may result in infection. Effects on the platelets and erythroid series are much less marked. Careful hematologic monitoring is required and since patients with HIV infection are immunocompromised, patients must be observed carefully for evidence of intercurrent or opportunistic infections.
Special attention must be given to the potential cardiac toxicity of Daunoxome. Although there is no reliable means of predicting congestive heart failure, cardiomyopathy induced by anthracyclines is usually associated with a decrease of the left ventricular ejection fraction (LVEF). Cardiac function should be evaluated in each patient by means of a history and physical examination before each course of Daunoxome and determination of LVEF should be performed at total cumulative doses of Daunoxome of 320 mg/m2, and every 160 mg/m2 thereafter.
Patients who have received prior therapy with anthracyclines (doxorubicin > 300 mg/m2 or equivalent), have pre-existing cardiac disease, or have received previous radiotherapy encompassing the heart may be less "cardiac" tolerant to treatment with Daunoxome. Therefore, monitoring of LVEF at cumulative DaunoXome doses should occur prior to therapy and every 160 mg/m2 of Daunoxome.
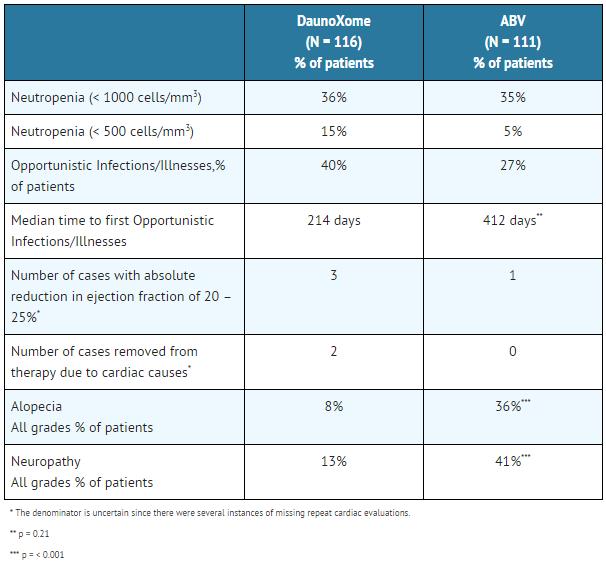
In patients with Kaposi's sarcoma, congestive heart failure has been reported in one patient at a cumulative dose of 340 mg/m2 of Daunoxome. In eight Kaposi's sarcoma patients, LVEF decreases were reported at cumulative doses ranging from 200 mg/m2 to 2100 mg/m2 (median dose 320 mg/m2) of Daunoxome. In clinical studies in malignancies other than Kaposi's sarcoma and treated with doses of Daunoxome greater than the recommended dose of 40 mg/m2, congestive heart failure has been reported at a cumulative dose as low as 200 mg/m2 of Daunoxome; seven patients have been reported with LVEF decreases. The proportion of patients at risk for cardiotoxicity is unknown because the denominator is uncertain since there were several instances of missing repeat cardiac evaluations.
A triad of back pain, flushing, and chest tightness has been reported in 13.8% of the patients (16/116) treated with Daunoxome in the randomized clinical trial and in 2.7% of treatment cycles (27/994). This triad generally occurs during the first five minutes of the infusion, subsides with interruption of the infusion, and generally does not recur if the infusion is then resumed at a slower rate. This combination of symptoms appears to be related to the lipid component of Daunoxome, as a similar set of signs and symptoms has been observed with other liposomal products not containing daunorubicin.
Daunorubicin has been associated with local tissue necrosis at the site of drug extravasation. Although no such local tissue necrosis has been observed with Daunoxome, care should be taken to ensure that there is no extravasation of drug when Daunoxome is administered.
Dosage should be reduced in patients with impaired hepatic function
Adverse Reactions
Clinical Trials Experience
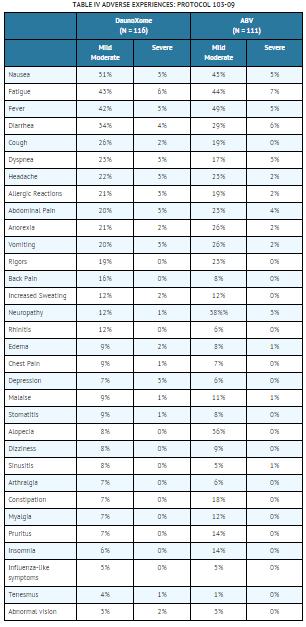
Daunoxome contains daunorubicin, encapsulated within a liposome. Conventional daunorubicin has acute myelosuppression as its dose limiting side effect, with the greatest effect on the granulocytic series. In addition, daunorubicin causes alopecia, and nausea and vomiting in a significant number of patients treated. Extravasation of conventional daunorubicin can cause severe local tissue necrosis. Chronic therapy at total doses above 300 mg/m2 causes a cumulative-dose-related cardiomyopathy with congestive heart failure.
Administered as DaunoXome, daunorubicin has substantially altered pharmacokinetics and some differences in toxicity. The most important acute toxicity of Daunoxome remains myelosuppression, principally of the granulocytic series, with much less marked effects on the platelets and erythroid series.
In an open-label, randomized, controlled clinical trial conducted in 13 centers in the U.S.A. and Canada in advanced HIV-related Kaposi's sarcoma, two treatment regimens were compared as first line cytotoxic therapy: DaunoXome and ABV (doxorubicin (Adriamycin®), bleomycin, and vincristine). All drugs were administered intravenously every 2 weeks. The safety data presented below include all reported or observed adverse experiences, including those not considered to be drug related. Patients with advanced HIV-associated Kaposi's sarcoma are seriously ill due to their underlying infection and are receiving several concomitant medications including potentially toxic antiviral and antiretroviral agents. The contribution of the study drugs to the adverse experience profile is therefore difficult to establish.
A triad of back pain, flushing and chest tightness was reported in 13.8% of the patients (16/116) treated with Daunoxome in the Phase III clinical trial and in 2.7% of treatment cycles (27/994). Most of the episodes were mild to moderate in severity (12% of patients and 2.5% of treatment cycles).
Mild alopecia was reported in 6% of patients treated with Daunoxome and moderate alopecia in 2% of patients. Mild nausea was reported in 35% of DaunoXome patients, moderate nausea in 16% of patients and severe nausea in 3% of patients. For patients treated with Daunoxome, mild vomiting was reported in 10%, moderate in 10%, and severe in 3% of patients. Although grade 3 – 4 injection site inflammation was reported in 2 patients treated with Daunoxome, no instances of local tissue necrosis were observed with extravasation.
Table IV is a listing of all the mild-moderate and severe adverse events reported on both treatment arms in Protocol 103-09 in ≥ 5% of Daunoxome patients.
Cardiovascular
Hot flushes, hypertension, palpitation, syncope, tachycardia. In other follow-up clinical trials of Daunoxome (daunorubicin citrate liposome injection) use in treatment of Kaposi's sarcoma or other malignancies, the following serious cardiac events were reported: Pericardial effusion, pericardial tamponade, ventricular extrasystoles, cardiac arrest, sinus tachycardia, atrial fibrillation, pulmonary hypertension, myocardial infarction, supraventricular tachycardia, and angina pectoris.
Digestive
Increased appetite, dysphagia, GI hemorrhage, gastritis, gingival bleeding, hemorrhoids, hepatomegaly, melena, dry mouth, tooth caries.
Hematologic and Lymphatic
Lymphadenopathy and splenomegaly
Metabolic and Nutritional
Dehydration and thirst
Neurologic
Amnesia, anxiety, ataxia, confusion, convulsions, emotional lability, abnormal gait, hallucination, hyperkinesia, hypertonia, meningitis, somnolence, abnormal thinking, tremor
Respiratory
Hemoptysis, hiccups, pulmonary infiltration, increased sputum
Skin and Hypersensitivy Reactions
Folliculitis, seborrhea, dry skin
Special Senses
Conjunctivitis, deafness, earache, eye pain, taste perversion, tinnitus
Urogenital
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Daunorubicin liposome in the drug label.
Drug Interactions
In the patient population studied, Daunoxome has been administered to patients receiving a variety of concomitant medications (e.g., antiretroviral agents, antiviral agents, anti-infective agents). Although interactions of Daunoxome (daunorubicin citrate liposome injection) with other drugs have not been observed, no systematic studies of interactions have been conducted.
Use in Specific Populations
Pregnancy
Daunoxome can cause fetal harm when administered to a pregnant woman. DaunoXome was administered to rats on gestation days 6 through 15 at 0.3, 1.0 or 2.0 mg/kg/day, (about 1/20th, 1/6th, or 1/3rd the recommended human dose on a mg/m2 basis). DaunoXome produced severe maternal toxicity and embryolethality at 2.0 mg/kg/day and was embryotoxic and caused fetal malformations (anophthalmia, microphthalmia, incomplete ossification) at 0.3 mg/kg/day. Embryotoxicity was characterized by increased embryo-fetal deaths, reduced numbers of litters, and reduced litter sizes.
There are no studies of Daunoxome in pregnant women. If Daunoxome is used during pregnancy, or if the patient becomes pregnant while taking Daunoxome, the patient must be warned of the potential hazard to the fetus. Patients should be advised to avoid becoming pregnant while taking Daunoxome.
Labor and Delivery
There is no FDA guidance on use of Daunorubicin liposome during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Daunorubicin liposome with respect to nursing mothers.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Safety and effectiveness in the elderly have not been established.
Gender
There is no FDA guidance on the use of Daunorubicin liposome with respect to specific gender populations.
Race
There is no FDA guidance on the use of Daunorubicin liposome with respect to specific racial populations.
Renal Impairment
Safety has not been established in patients with pre-existing hepatic or renal dysfunction.
Hepatic Impairment
There is no FDA guidance on the use of Daunorubicin liposome in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Daunorubicin liposome in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Daunorubicin liposome in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
The primary toxicity of Daunoxome is myelosuppression, especially of the granulocytic series, which may be severe, and associated with fever and may result in infection. Effects on the platelets and erythroid series are much less marked. Careful hematologic monitoring is required and since patients with HIV infection are immunocompromised, patients must be observed carefully for evidence of intercurrent or opportunistic infections.
Patients who have received prior therapy with anthracyclines (doxorubicin > 300 mg/m2 or equivalent), have pre-existing cardiac disease, or have received previous radiotherapy encompassing the heart may be less "cardiac" tolerant to treatment with DaunoXome. Therefore, monitoring of LVEF at cumulative Daunoxome doses should occur prior to therapy and every 160 mg/m2 of Daunoxome.
Overdosage
The symptoms of acute overdosage are increased severities of the observed dose-limiting toxicities of therapeutic doses of Daunoxome, myelosuppression (especially granulocytopenia), fatigue, and nausea and vomiting.
Pharmacology
Mechanism of Action
Daunoxome is a liposomal preparation of daunorubicin formulated to maximize the selectivity of daunorubicin for solid tumors in situ. While in the circulation, the Daunoxome formulation helps to protect the entrapped daunorubicin from chemical and enzymatic degradation, minimizes protein binding, and generally decreases uptake by normal (non-reticuloendothelial system) tissues. The specific mechanism by which Daunoxome is able to deliver daunorubicin to solid tumors in situ is not known. However, it is believed to be a function of increased permeability of the tumor neovasculature to some particles in the size range of Daunoxome. In animal studies, daunorubicin has been shown to accumulate in tumors to a greater extent when administered as Daunoxome than when administered as daunorubicin. Once within the tumor environment, daunorubicin is released over time enabling it to exert its antineoplastic activity.
Structure
Daunoxome (daunorubicin citrate liposome injection) is a sterile, pyrogen-free, preservative-free product in a single use vial for intravenous infusion.
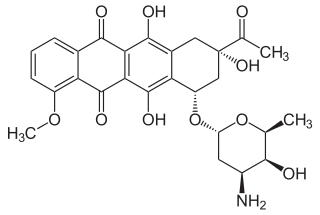
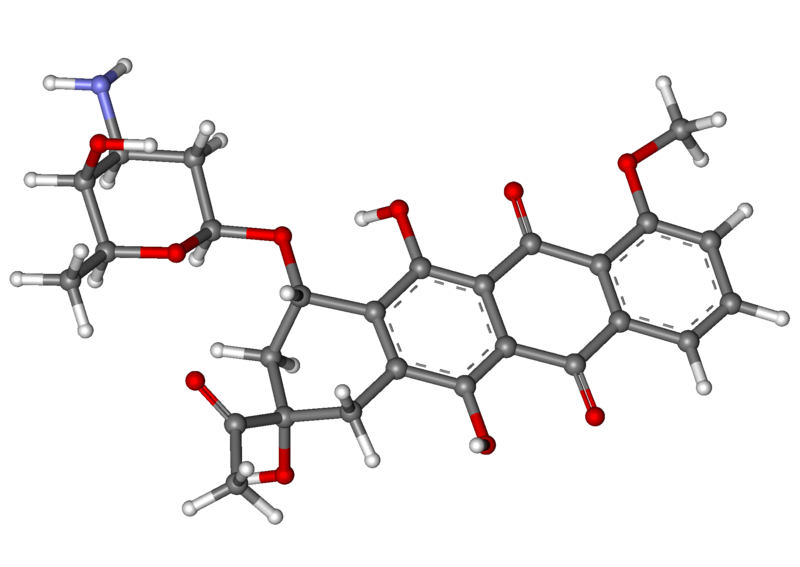
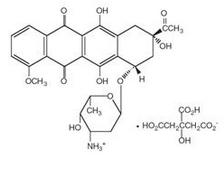
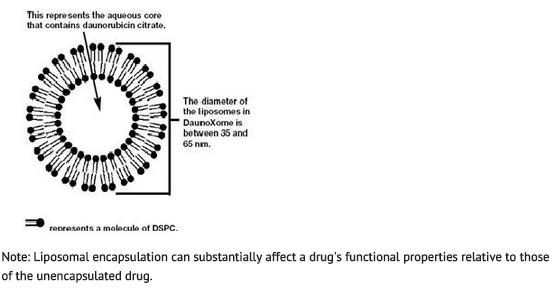
Daunoxome contains an aqueous solution of the citrate salt of daunorubicin encapsulated within lipid vesicles (liposomes) composed of a lipid bilayer of distearoylphosphatidylcholine and cholesterol (2:1 molar ratio), with a mean diameter of about 45 nm. The lipid to drug weight ratio is 18.7:1 (total lipid:daunorubicin base), equivalent to a 10:5:1 molar ratio of distearoylphosphatidylcholine:cholesterol:daunorubicin. Daunorubicin is an anthracycline antibiotic with antineoplastic activity, originally obtained from Streptomyces peucetius. Daunorubicin has a 4-ring anthracycline moiety linked by a glycosidic bond to daunosamine, an amino sugar. Daunorubicin may also be isolated from Streptomyces coeruleorubidus and has the following chemical name: (8S-cis)-8-acetyl-10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride.
Daunorubicin citrate has the following chemical structure:
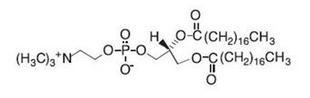
DSPC (distearoylphosphatidylcholine) has the following chemical structure:
The following represents the idealized, spherical morphology of a liposome:
Each vial contains daunorubicin citrate equivalent to 50 mg of daunorubicin base, encapsulated in liposomes consisting of 704 mg distearoylphosphatidylcholine and 168 mg cholesterol. The liposomes encapsulating daunorubicin are dispersed in an aqueous medium containing 2,125 mg sucrose, 94 mg glycine, and 7 mg calcium chloride dihydrate in a total volume of 25 mL/vial. The pH of the dispersion is between 4.9 and 6.0. The liposome dispersion should appear red and translucent.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Daunorubicin liposome in the drug label.
Pharmacokinetics
Following intravenous injection of Daunoxome, plasma clearance of daunorubicin shows monoexponential decline. The pharmacokinetic parameter values for total daunorubicin following a single 40 mg/m2 dose of Daunoxome administered over a 30 – 60 minute period to patients with AIDS-related Kaposi's sarcoma and following a single rapid intravenous, 80 mg/m2 dose of conventional daunorubicin to patients with disseminated solid malignancies are shown in Table I.
The plasma pharmacokinetics of Daunoxome differ significantly from the results reported for conventional daunorubicin hydrochloride. Daunoxome has a small steady-state volume of distribution of 6.4 L, (probably because it is confined to vascular fluid volume), and clearance of 17 mL/min. These differences in the volume of distribution and clearance result in a higher daunorubicin exposure (in terms of plasma AUC) from Daunoxome than with conventional daunorubicin hydrochloride. The apparent elimination half-life of Daunoxome (daunorubicin citrate liposome injection) is 4.4 hours, far shorter than that of daunorubicin, and probably represents a distribution half-life. Although preclinical biodistribution data in animals suggest that Daunoxome crosses the normal blood-brain barrier, it is unknown whether Daunoxome crosses the blood-brain barrier in humans.
Metabolism: Daunorubicinol, the major active metabolite of daunorubicin, was detected at low levels in the plasma following intravenous administration of Daunoxome.
No formal assessments of pharmacokinetic drug–drug interactions between DaunoXome and other agents have been conducted.
Special Populations: The pharmacokinetics of DaunoXome have not been evaluated in women, in different ethnic groups, or in subjects with renal and hepatic insufficiency.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No carcinogenesis, mutagenesis, or impairment of fertility studies were conducted with DaunoXome.
Carcinogenesis: Carcinogenicity and mutagenicity studies have been conducted with daunorubicin, the active component of Daunoxome. A high incidence of mammary tumors was observed about 120 days after a single intravenous dose of 12.5 mg/kg daunorubicin in rats (about 2 times the human dose on a mg/m2 basis). Mutagenesis: Daunorubicin was mutagenic in in vitro tests (Ames assay, V79 hamster cell assay), and clastogenic in in vitro (CCRF-CEM human lymphoblasts) and in in vivo (SCE assay in mouse bone marrow) tests. Impairment of Fertility: Daunorubicin intravenous doses of 0.25 mg/kg/day (about 8 times the human dose on a mg/m2 basis) in male dogs caused testicular atrophy and total aplasia of spermatocytes in the seminiferous tubules.
Clinical Studies
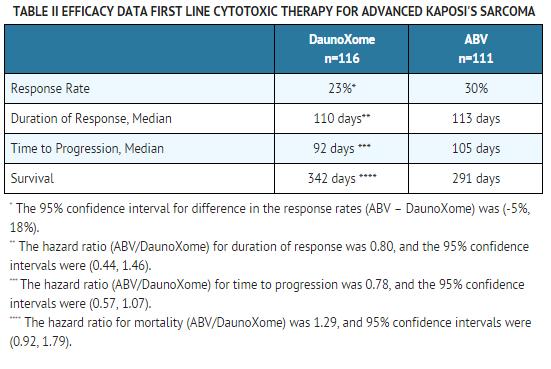
Clinical Study: In an open-label, randomized, controlled clinical study conducted at 13 centers in the U.S.A. and Canada in advanced (25 or more mucocutaneous lesions; the development of 10 or more lesions in a one month period of time; symptomatic visceral involvement; or tumor-associated edema) HIV-related Kaposi's sarcoma, two treatment regimens were compared as first line cytotoxic therapy: DaunoXome 40 mg/m2and ABV (doxorubicin (Adriamycin®1) 10 mg/m2, bleomycin 15 U, and vincristine 1.0 mg). All drugs were administered intravenously every 2 weeks. Responses were assessed using the AIDS Clinical Trials Group Oncology Committee of the National Institute of Allergy and Infectious Diseases (ACTG) criteria (a response required at least one of any of the following for at least 28 days: a. ≥ 50% reduction in the number; b. ≥ 50% reduction in the sums of the products of the largest perpendicular diameters of bidimensionally measurable marker lesions; or c. complete flattening of ≥ 50% of all previously raised lesions). Table II summarizes the efficacy results.
1. Adriamycin is a registered trademark of Pharmacia & Upjohn Co., Kalamazoo, MI.
Twenty of the 33 ABV responders responded to therapy by criteria more stringent than flattening of lesions (i.e., shrinkage of lesions and/or reduction in the number of lesions). Eleven of the 27 DaunoXome responders responded to therapy by criteria other than flattening of lesions. Photographic evidence of tumor response to DaunoXome and ABV was comparable across all anatomic sites (e.g., face, oral cavity, trunk, legs, and feet).
How Supplied
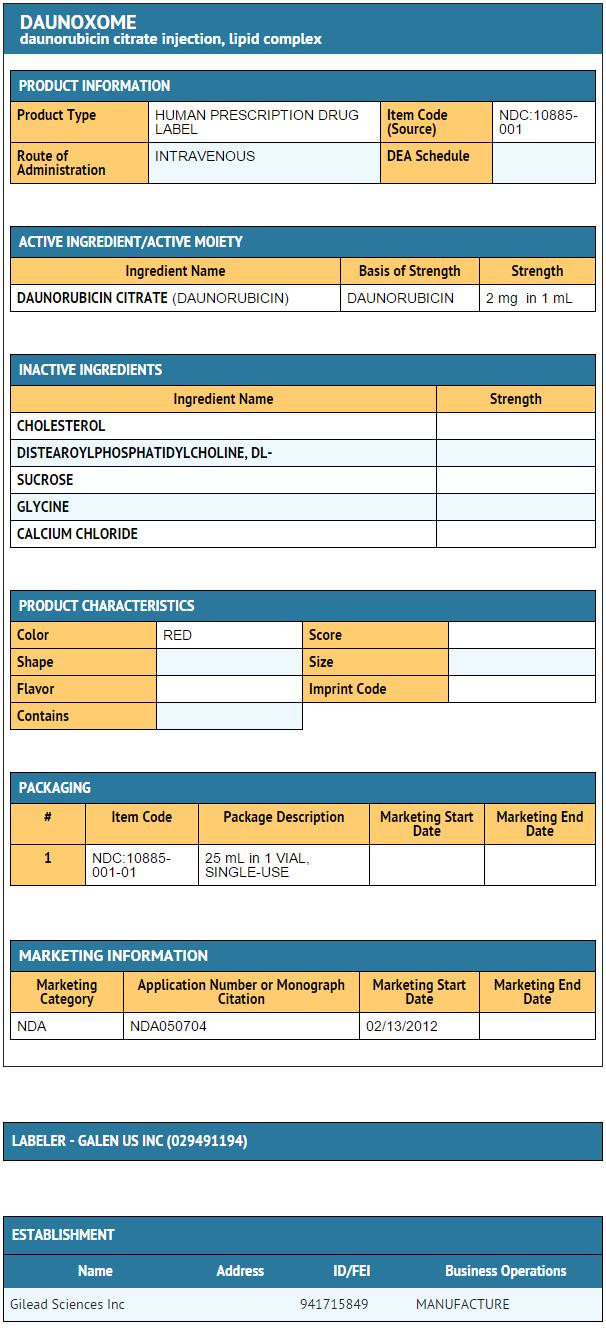
- Daunoxome is a translucent, red, liposomal dispersion supplied in single use vials, each sealed with a synthetic rubber stopper and aluminum sealing ring with a plastic cap. Daunoxome provides daunorubicin citrate equivalent to 50 mg of daunorubicin base, at a concentration of 2 mg/mL.
Daunoxome is supplied under NDC 10885-001-01 for a single unit pack.
Images
Package and Label Display Panel
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Daunorubicin liposome in the drug label.
Precautions with Alcohol
- Alcohol-Daunorubicin liposome interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Daunoxome®
Look-Alike Drug Names
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Daunorubicin liposome
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Daunorubicin liposome |Label Name=Daunorubicin liposome11.png
}}
{{#subobject:
|Label Page=Daunorubicin liposome |Label Name=Daunorubicin liposome11.png
}}