DURACLON labels and packages
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
For patient information, click here.
Labels and Packages
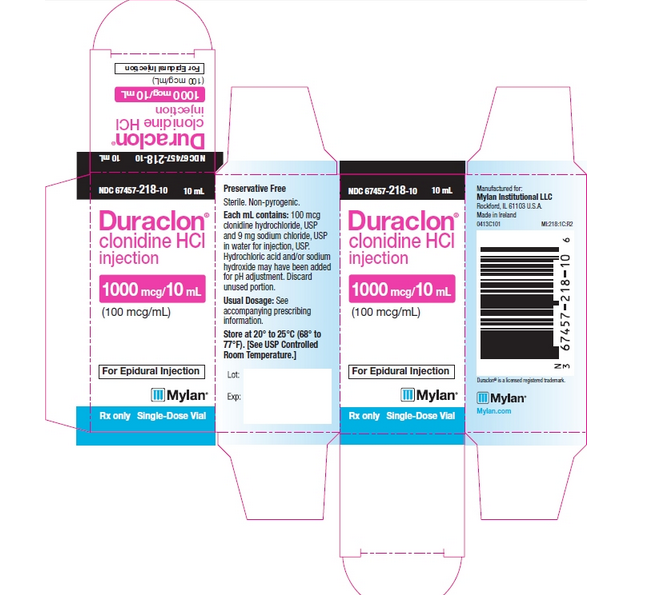
NDC 67457-218-10 10 mL
Duraclon® clonidine HCl injection
1000 mcg/10 mL (100 mcg/mL)
For Epidural Injection
Rx only Single-Dose Vial
Preservative Free
Sterile. Non-pyrogenic.
Each mL contains: 100 mcg clonidine hydrochloride, USP and 9 mg sodium chloride, USP in water for injection, USP.
Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Discard unused portion.
Usual Dosage: See accompanying prescribing information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured for: Mylan Institutional LLC Rockford, IL 61103 U.S.A. Made in Ireland 0414C101 MI:218:1C:R2
Duraclon® is a licensed registered trademark.
Mylan.com
 |
PRINCIPAL DISPLAY PANEL
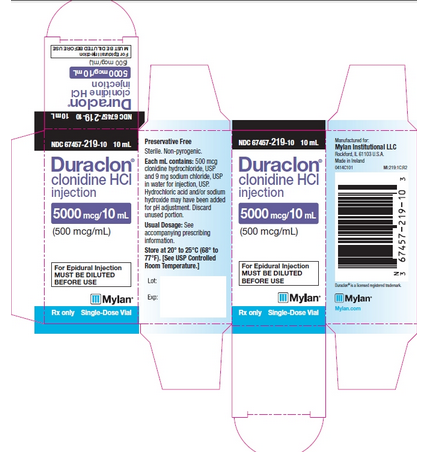
NDC 67457-219-10 10 mL
Duraclon® clonidine HCl injection
5000 mcg/10 mL (500 mcg/mL)
For Epidural Injection MUST BE DILUTED BEFORE USE
Rx only Single-Dose Vial
Preservative Free
Sterile. Non-pyrogenic.
Each mL contains: 500 mcg clonidine hydrochloride, USP and 9 mg sodium chloride, USP in water for injection, USP.
Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Discard unused portion.
Usual Dosage: See accompanying prescribing information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured for: Mylan Institutional LLC Rockford, IL 61103 U.S.A. Made in Ireland 0413C101 MI:219:1C:R2
Duraclon® is a licensed registered trademark.
Mylan.com
 |
References
- ↑ "DURACLON (CLONIDINE HYDROCHLORIDE) INJECTION, SOLUTION [MYLAN INSTITUTIONAL LLC]". Retrieved 6 February 2014.