Cyanate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
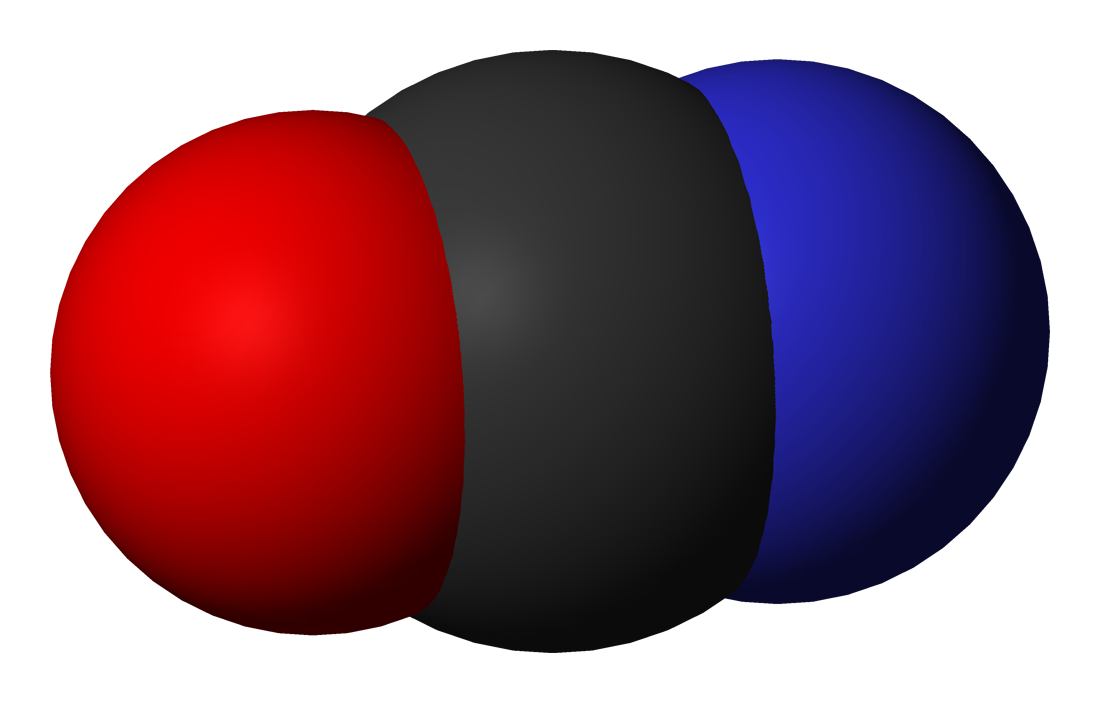
The cyanate ion is an anion consisting of one oxygen atom, one carbon atom, and one nitrogen atom, [OCN]−, in that order, and possesses 1 unit of negative charge, borne mainly by the nitrogen atom. In organic compounds the cyanate group is a functional group.
The structure of cyanate can be considered to resonate between two canonical forms:
The resonance hybrid resulting from these two contributory structures can be represented as
The cyanate ion is isoelectronic with carbon dioxide, and so shares its linear shape.
The cyanate ion is an ambident nucleophile in nucleophilic substitution because it can react to form an alkyl cyanate R-OCN (exception) or an alkyl isocyanate R-NCO (rule). Aryl cyanates (C6H5OCN) can be formed by a reaction of phenol with cyanogen chloride (ClCN) in the presence of a base.
Cyanates are salts or esters of cyanic acid, for example potassium cyanate (KOCN) or methyl cyanate.
The cyanate ion is relatively non-toxic in comparison with cyanides. Use of this fact is made in cyanide decontamination processes where a permanganate oxidation converts toxic cyanide to safer cyanate.
The fulminate ion [ONC]− has the same chemical formula but a different structure — it is a structural isomer of cyanate.