Clindamycin hydrochloride warnings
| Clindamycin hydrochloride |
|---|
| CLEOCIN HYDROCHLORIDE® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
| Indications and Usage |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| How Supplied |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
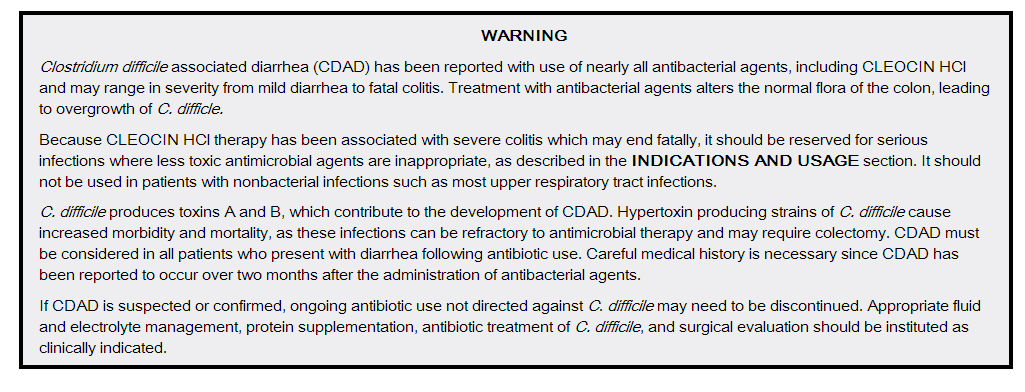
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including CLEOCIN HCl, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
Usage in Meningitis
Since clindamycin does not diffuse adequately into the cerebrospinal fluid, the drug should not be used in the treatment of meningitis.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/50162s082,50441s045,50639s013lbl.pdf