Ciliary ganglion
Template:Infobox Nerve Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
The ciliary ganglion is a parasympathetic ganglion located in the posterior orbit. It measures 1- 2 millimeters in diameter and contains approximately 2,500 neurons[1]. Preganglionic axons from the Edinger-Westphal nucleus form synapses with these cells. The postganglionic axons innervate two eye muscles:
- the sphincter pupillae constricts the pupil.
- the ciliaris muscle changes the shape of the lens.
Both of these muscles are involuntary – they are controlled by the autonomic nervous system.
Anatomy
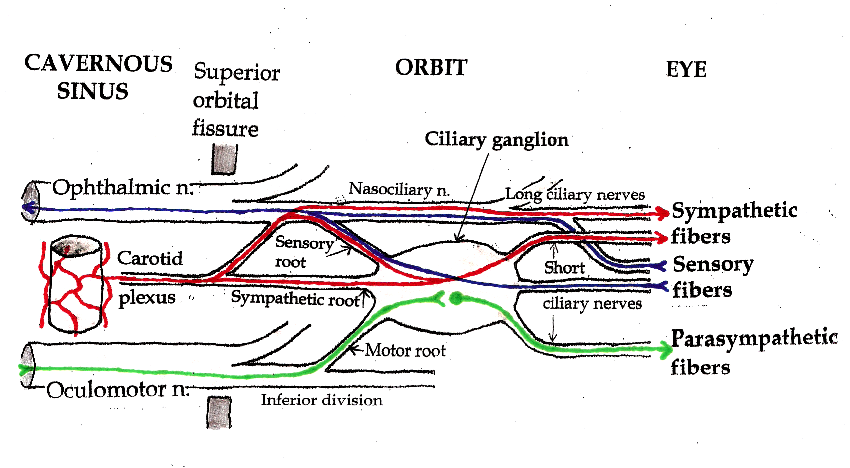
Three types of nerve fibers run through the ciliary ganglion: parasympathetic fibers, sympathetic fibers and sensory fibers. Only parasympathetic fibers form synapses in the ganglion. The other two types of nerve fibers simply pass through. In classical anatomy, the ciliary ganglion is said to have three “roots:” a parasympathetic motor root, a sympathetic root and a sensory root.
Motor root
The ciliary ganglion is a parasympathetic ganglion. Incoming parasympathetic nerve fibers form synapses with the dendrites of nerve cells within the ganglion. However, the ciliary ganglion is not simply a relay station connecting preganglionic to postganglionic nerve fibers. There are roughly twice as many incoming parasympathetic fibers as outgoing parasympathetic fibers. Neural processing occurs as incoming signals converge onto target neurons.
Presynaptic parasympathetic fibers originate in the Edinger-Westphal nucleus, the parasympathetic motor nucleus associated with the oculomotor nucleus in the brainstem. Axons from the Edinger-Westphal nucleus and the oculomotor nucleus run together in the brainstem and exit together as the oculomotor nerve (cranial nerve III). The oculomotor nerve passes through the cavernous sinus and enters the orbit through the superior orbital fissure. It divides into branches that innervate the levator palpebrae superioris and four of the six extraocular muscles. Parasympathetic fibers initially run in the inferior division of the oculomotor nerve. They exit as one or two short “motor roots” that synapse in the ciliary ganglion.
Postsynaptic parasympathetic fibers leave the ciliary ganglion in multiple (six to ten) short ciliary nerves. These nerves enter the posterior aspect of the eyeball to innervate the sphincter pupillae and ciliaris muscles. The sphincter pupillae constricts the iris. The ciliaris muscle changes the shape of the lens, allowing the eye to focus on nearby objects (accommodation). Neither of these muscles is under voluntary control.
Sympathetic root
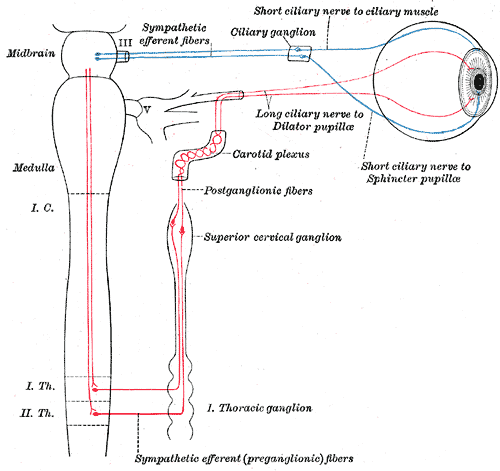
The “sympathetic root” of the ciliary ganglion contains postganglionic sympathetic fibers whose cell bodies are located in the superior cervical ganglion. Their axons ascend with the internal carotid artery as a plexus of nerves, the carotid plexus. Sympathetic fibers innervating the eye separate from the carotid plexus within the cavernous sinus. They run forward through the superior orbital fissure and merge with the long ciliary nerves (branches of the nasociliary nerve) and the short ciliary nerves (from the ciliary ganglion). Sympathetic fibers in the short ciliary nerves pass through the ciliary ganglion without forming synapses.
Preganglionic sympathetic fibers originate from neurons in the intermediolateral column of the thoracic spinal cord, at the level of T1 and T2. They form synapses in the superior cervical ganglion. The ratio of incoming to outgoing fibers (the “convergence”) in this ganglion is approximately100:1. Sympathetic motor neurons in the spinal cord are controlled by supranuclear pathways that descend through the brainstem and spinal cord. Interruption of the sympathetic chain at any level (from the brainstem to the ciliary ganglion) will produce pupillary constriction (miosis) and eyelid droop (ptosis) – the classic Horner syndrome.
Sympathetic fibers from the superior cervical ganglion innervate blood vessels (vasoconstriction), sweat glands, and four eye muscles: the dilator pupillae, the superior tarsal muscle, the inferior tarsal muscle and the orbitalis.
The dilator pupillae dilates the pupil; its action is antagonistic to the sphincter pupillae. Pupil size is therefore under the dual control of sympathetic and parasympathetic nerves.
The superior tarsal muscle elevated the upper eyelid. The levator palpebrae superioris, which is innervated by a branch of the oculomotor nerve, also elevates the upper eyelid. Eyelid elevation is therefore under both voluntary and involuntary control. Interruption of either pathway will result in eyelid droop (ptosis).
The other two eye muscles with sympathetic innervation (the inferior tarsal muscle and the orbitalis) are vestigial in humans. They are variable and often incompletely developed.
Sensory root
Sensory fibers from the eyeball (the cornea, iris and ciliary body) run posteriorly through the short ciliary nerves and pass through the ciliary ganglion without forming synapses. They leave the ciliary ganglion in the “sensory root”, which joins the nasociliary nerve. The nasociliary nerve is a branch of the ophthalmic nerve, one of the three branches (V1) of the trigeminal nerve. The trigeminal nerve is the main sensory nerve of the face.
Sensory fibers from other parts of the eye run through the long ciliary nerves and other peripheral branches of the ophthalmic nerve. The exact distribution of sensory fibers, like the distribution of sympathetic fibers, is anatomically variable. There are alternate pathways to the eye for both sympathetic and sensory fibers, and the precise anatomy varies from person to person. Since the result is the same regardless of how the fibers reach the eye, the presence of sympathetic and sensory fibers in the ciliary ganglion (the contributions of the “sensory” and “sympathetic” roots) is of no functional significance.
Diseases
Adie tonic pupil
Diseases of the ciliary ganglion produce a tonic pupil[2]. This is a pupil that does not react to light (it is “fixed”) and has an abnormally slow and prolonged response to attempted near vision (accommodation).
When a patient with an Adie pupil attempts to focus on a nearby object, the pupil (which would normally constrict rapidly) constricts slowly. On close inspection, the constrcted pupil is not perfectly round. When the patient focuses on a more distant object (say the far side of the room), the pupil (which would normally dilate immediately) remains constricted for several minutes, and then slowly dilates back to the expected size.
Tonic pupils are fairly common – they are seen in roughly 1 out of every 500 people. A patient with anisocoria (one pupil bigger than the other) whose pupil does not react to light (does not constrict when exposed to bright light) most likely has Adie syndrome – idiopathic degeneration of the ciliary ganglion.
Physiology
The strange behavior of tonic pupils was first explained by Irene Loewenfeld in 1979. The ciliary ganglion contain many more nerve fibers directed to the ciliary muscle than nerve fibers directed to the constrictor pupillae – roughly twenty times more. The ciliary muscle is also more massive than the constrictor pupillae, again by a factor of twenty. Based on these observations, Loewenfeld proposed an explanation of the tonic pupil. She noted that pathological destruction of nerve cells in the ciliary ganglion that is found in all cases of Adie pupil. In her own words[3] :
- Let’s say that in a given fresh Adie’s pupil, a random 70% of the cells in the ciliary ganglion stop working; and that, in a couple of months, these neurons re-grow and randomly re-innervate both intraocular sphincters (the ciliary muscle and the iris sphincter). Some parasympathetic light-reaction neurons that were originally destined for the iris sphincter will end up innervating the ciliary muscle. But there will not be enough of them to budge that big muscle, so there will be no detectable accommodation with exposure to light. The other way around, it is a different story. There will be plenty of accommodative neurons re-growing into the iris sphincter, and it won’t take very many of them to make a little muscle like the iris sphincter contract. This means that every time the patient accommodates her gaze to a near object, some of the innervation to the ciliary muscle will spill over into the iris and constrict the pupil.
Loewenfeld’s theory is now generally accepted. It explains the defining features of a tonic pupil:
- (1) The pupil does not react to light. The original light-reaction neurons have been destroyed.
- (2) Tonic constriction with attempted near vision. Aberrant regeneration of nerve fibers intended for the ciliary muscle causes abnormal, tonic contraction of the pupil with accommodation.
- (3) Segmental iris constriction. When carefully examined under magnification, the iris does not constrict uniformly with attempted near vision. Only the re-innervated segments contract, producing a slightly irregular contour to the pupil.
- (4) Denervation supersensitivity. Like any denervated muscle, the iris becomes supersensitive to its normal neurotransmitter (in this case, acetylcholine). Very weak solutions of cholinergic substances such as pilocarpine (that have no effect on the normal iris) cause the denervated iris to constrict.
Tonic pupils are usually due to Adie syndrome, but other diseases can denervate the ciliary ganglion. Peripheral neuropathies (such as diabetic neuropathy) occasionally produce tonic pupils. Herpes zoster virus can attack the ciliary ganglion. Trauma to the orbit can damage the short ciliary nerves. Anything that denervates the ciliary ganglion will produce a tonic pupil due to aberrant nerve regeneration.
Adie syndrome
Adie syndrome[4] is tonic pupil plus absent deep tendon reflexes. Adie syndrome is a fairly common, benign, idiopathic neuropathy that selectively affects the ciliary ganglion and the spinal cord neurons involved in deep tendon reflex arcs. It usually develops in middle age, although it can occur in children. A variant of Adie syndrome, Ross syndrome, affects sweating as well.
Early in the course of Adie syndrome (when the cells of the ciliary ganglion have been destroyed, but before regeneration has occurred) the pupil will be fixed and dilated. The sphincter pupillae will be paralyzed. There will be no response to accommodation – the ciliary muscle is also paralyzed.
With aberrant nerve regeneration, the pupil will remain fixed, but it will constrict with attempted near vision. The constriction will be abnormal (“tonic”).
Late in the course of Adie syndrome, the pupil becomes small (as all pupils do with old age). It will still be “fixed” (it will not constrict to bright light) and it will continue to show abnormal, tonic constriction with attempted near vision.
Light-near dissociation
The Adie pupil does not react to light, but it does react to accommodation. This is an example of “light-near dissociation”. All other causes of light-near dissociation involve the brainstem. They do not involve the ciliary ganglion, and they do not produce a tonic pupil. Irene Loewenfeld is generally credited for being the first physiologist to make this distinction.
The brainstem causes of light-near dissociation include Argyll Robertson pupil and Parinaud syndrome. These are discussed elsewhere in Wikipedia.
References
- ↑ Perez GM, Keyser RB. Cell Body Counts in Human Ciliary Ganglia. Investigative Ophthalmology & Visual Science 27:1428-1431, 1986
- ↑ Kawasaki A. Physiology, assessment, and disorders of the pupil. Curr Opin Ophthalmol 10:394-400, 1999
- ↑ Thompson HS, Kardon Rh. Irene Loewenfeld, PhD Physiologist of the Pupil. J Neuroophthalmol 26:139-148, 2006
- ↑ Thompson HS. Adie’s Syndrome: Some New Observations. Tr. Am. Ophth. Soc. LXXV:597-626, 1977
Bibliography
- Blumenfeld H. Neuroanatomy Through Clinical Cases. Sinauer Associates, 2002
- Brodal P. The Central Nervous System, 3rded. Oxford University Press, 2004
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy, 2nd ed. Wiley-Interscience, 2005
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed. McGraw-Hill, 2000
- Martin JH. Neuroanatomy Text and Atlas, 3rd ed. McGraw-Hill, 2003
- Patten J. Neurological Differential Diagnosis, 2nd ed. Springer, 1996
- Ropper, AH, Brown RH. Victor’s Principles of Neurology, 8th ed. McGraw-Hill, 2005
- Standring S (ed.) Gray’s Anatomy, 39th edition. Elsevier Churchill Livingstone, 2005
- Wilson-Pauwels L, Akesson EJ, Stewart PA. Cranial Nerves: Anatomy and Clinical Comments. Decker, 1998
Additional images
-
Plan of oculomotor nerve.
-
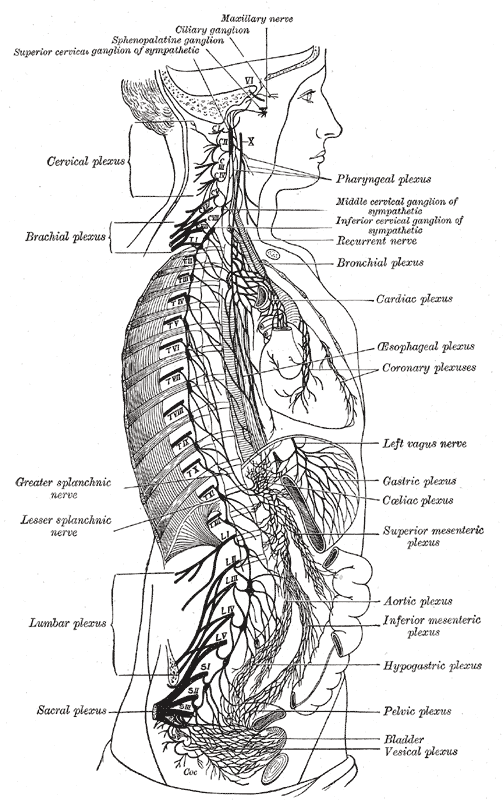
The right sympathetic chain and its connections with the thoracic, abdominal, and pelvic plexuses.
-
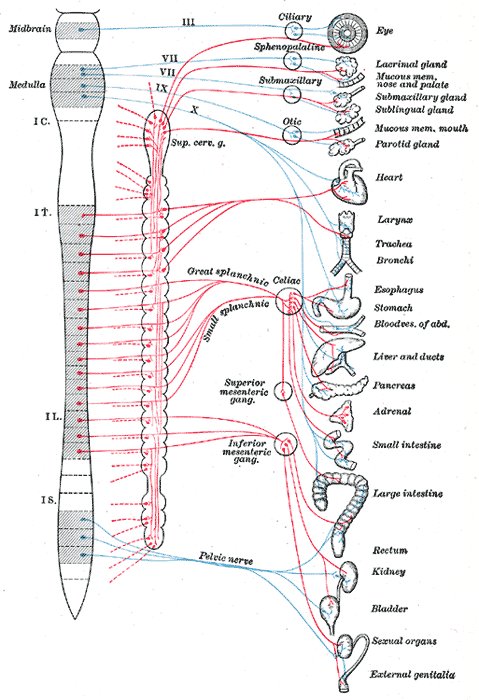
Diagram of efferent sympathetic nervous system.
-
Sympathetic connections of the ciliary and superior cervical ganglia.
External links
- Template:SUNYAnatomyFigs - "A deeper dissection of the right orbit from a superior approach."
- Template:UMichAtlas - "Branches of Trigeminal Nerve, Lateral View"
- Template:NormanAnatomy (Template:NormanAnatomyFig)
- Template:NormanAnatomy (Template:NormanAnatomyFig, Template:NormanAnatomyFig)