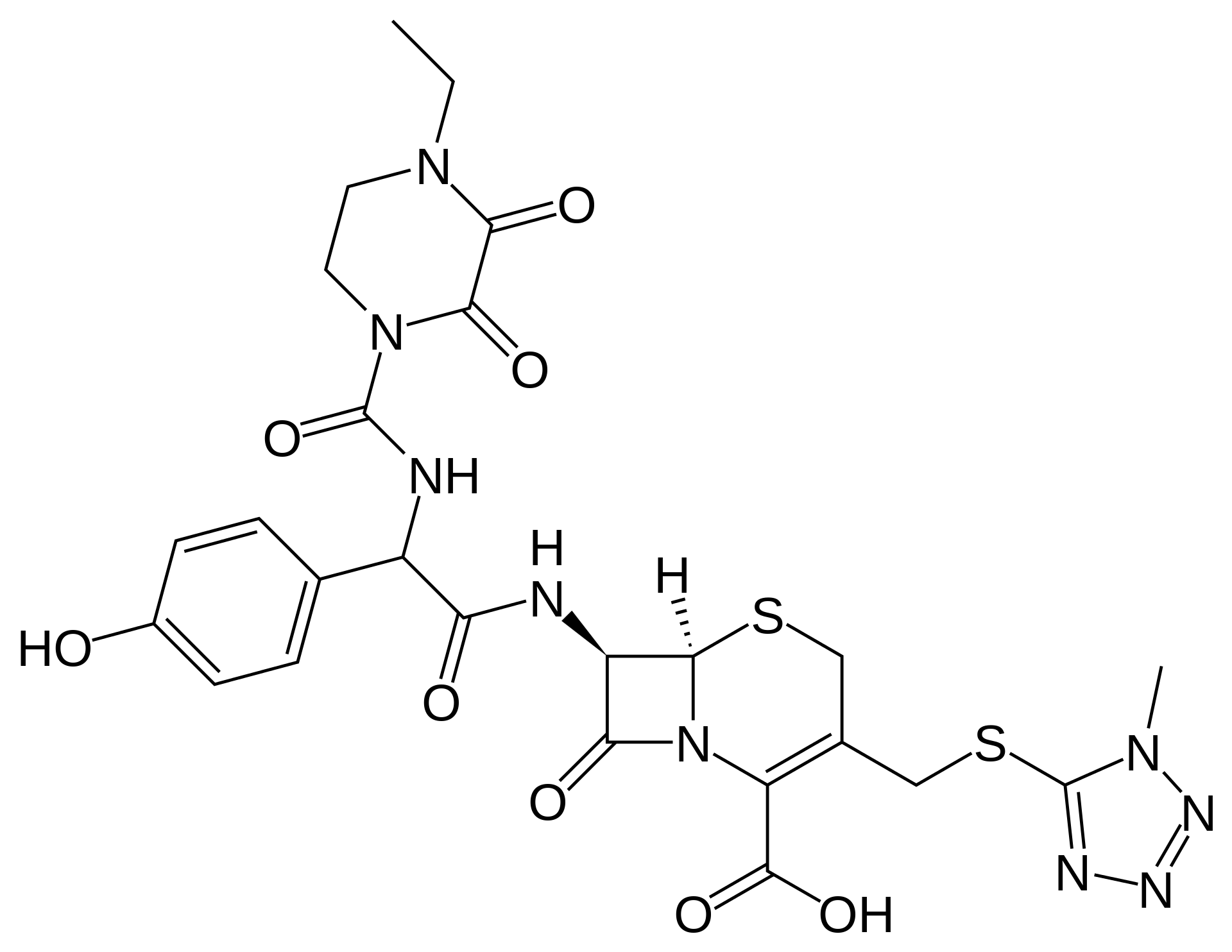
Cefoperazone
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Hepatic |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H27N9O8S2 |
| Molar mass | 645.67 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Cefoperazone |
|
Articles |
|---|
|
Most recent articles on Cefoperazone Most cited articles on Cefoperazone |
|
Media |
|
Powerpoint slides on Cefoperazone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cefoperazone at Clinical Trials.gov Clinical Trials on Cefoperazone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cefoperazone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cefoperazone Discussion groups on Cefoperazone Patient Handouts on Cefoperazone Directions to Hospitals Treating Cefoperazone Risk calculators and risk factors for Cefoperazone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cefoperazone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid, and also marked by Pharco B International under the name Cefazone and also marketed by Sigmatec Pharmaceuticals under the name Cefoperazone. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics. Cefina-SB is a combination of sulbactam and cefoperazone. Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis, and sulbactam acts as a beta-lactamase inhibitor, to increase the antibacterial activity of cefoperazone against beta-lactamase-producing organisms. In some countries, the combination is sold as Sulperazone. Gepach International markets this combination of cefoperazone with sulbactam under the brand name Bacperazone.In India and SriLanka (Cefoperazone-Sulbactam) is manufacterd by Pfizer under the brand name of Magnex/Magnex-Forte depending on the Cefoperazone-Sulbactam ratio.
Spectrum of bacterial susceptibility
Cefoperazone has a broad spectrum of activity and has been used to target bacteria responsible for causing infections of the respiratory and urinary tract, skin, and the female genital tract. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: 0.12 - 0.25 µg/ml
- Staphylococcus aureus: 0.125 - 32 µg/ml
- Streptococcus pneumoniae: ≤0.007 - 1 µg/ml[1][2]
Adverse effects
Cefoperazone contains an N-methylthiotetrazole (NMTT or 1-MTT) side chain. As the antibiotic is broken down in the body, it releases free NMTT, which can cause hypoprothrombinemia (likely due to inhibition of the enzyme vitamin K epoxide reductase) and a reaction with ethanol similar to that produced by disulfiram (Antabuse), due to inhibition of aldehyde dehydrogenase.[3]
References
- ↑ http://antibiotics.toku-e.com/antimicrobial_462_1.html
- ↑ http://www.toku-e.com/Assets/MIC/Cefoperazone%20sodium.pdf
- ↑ Stork CM (2006). "Antibiotics, antifungals, and antivirals". In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.). Goldfrank's toxicologic emergencies. New York: McGraw-Hill. p. 847. ISBN 0-07-143763-0. Retrieved 2009-07-03.
- Pages with script errors
- CS1 maint: Multiple names: editors list
- CS1 maint: Extra text: editors list
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Cephalosporin antibiotics
- Tetrazoles
- Piperazines
- Phenols
- Drug
- Lactams