Brompheniramine maleate and Pseudoephedrine hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Brompheniramine maleate and Pseudoephedrine hydrochloride is an anti-allergic agent with vasoconstrictor agent that is FDA approved for the treatment of nasal congestion due to the common cold, hay fever or other upper respiratory allergies, or associated with sinusitis, and symptoms due to hay fever (allergic rhinitis). Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies, or associated with sinusitis
- Temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily restores freer breathing through the nose
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Brompheniramine maleate and Pseudoephedrine hydrochloride in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Brompheniramine maleate and Pseudoephedrine hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Brompheniramine maleate and Pseudoephedrine hydrochloride in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Brompheniramine maleate and Pseudoephedrine hydrochloride in pediatric patients.
Contraindications
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Contraindications in the drug label.
Warnings
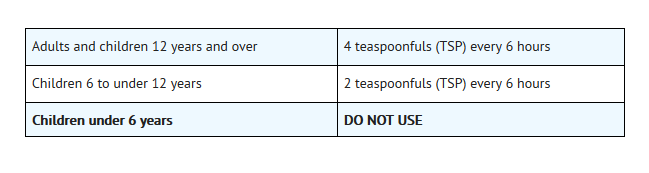
- Do not use in a child under 6 years of age
- Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- breathing problem such as emphysema or chronic bronchitis
- Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
- When using this product
- do not use more than directed
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- Stop use and ask a doctor if
- you get nervous, dizzy, or sleepless
- symptoms do not improve within 7 days or are accompanied by fever
- If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Brompheniramine maleate and Pseudoephedrine hydrochloride in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Brompheniramine maleate and Pseudoephedrine hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Brompheniramine maleate and Pseudoephedrine hydrochloride during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in geriatric settings.
Gender
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Brompheniramine maleate and Pseudoephedrine hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Do not take more than 4 doses in any 24-hour period
Monitoring
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Brompheniramine maleate and Pseudoephedrine hydrochloride and IV administrations.
Overdosage
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Mechanism of Action in the drug label.
Structure
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Structure in the drug label.
Pharmacodynamics
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Clinical Studies in the drug label.
How Supplied
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride How Supplied in the drug label.
Storage
- Store at room temperature 20°-25°C (68°-77°F).
Images
Drug Images
{{#ask: Page Name::Brompheniramine maleate and Pseudoephedrine hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
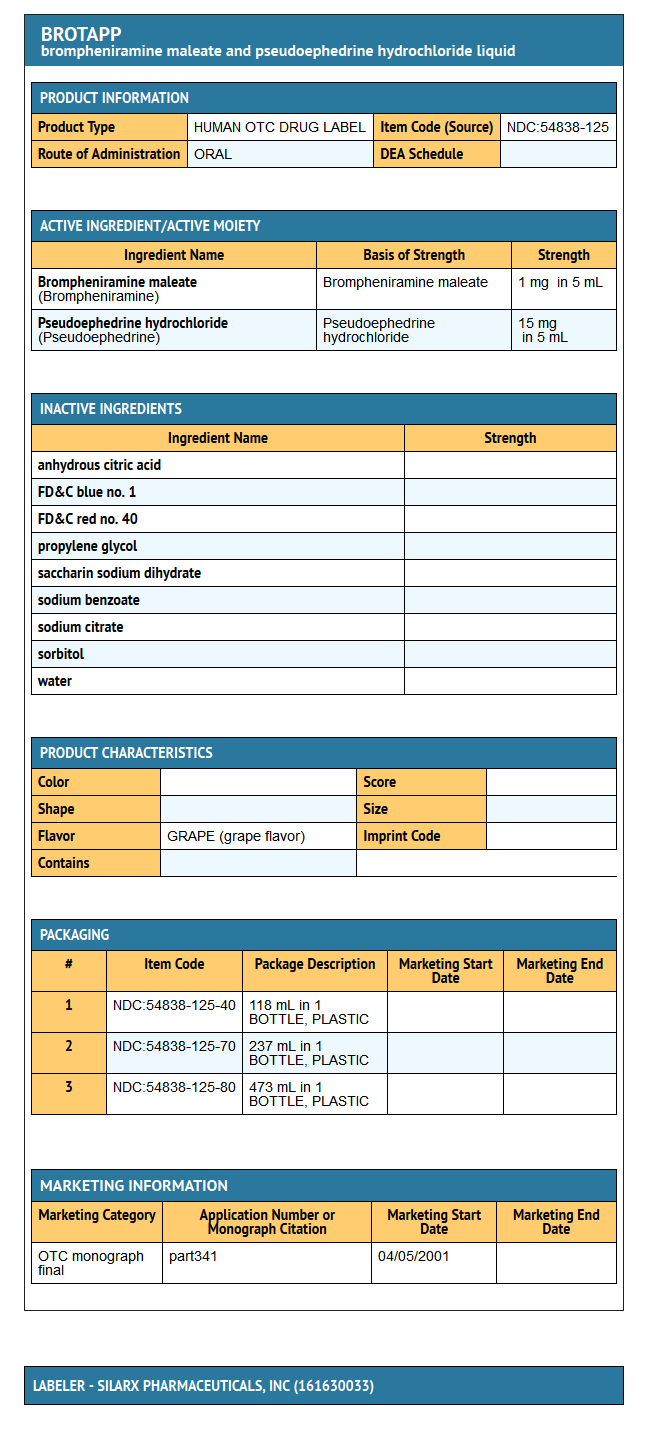
{{#ask: Label Page::Brompheniramine maleate and Pseudoephedrine hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Brompheniramine maleate and Pseudoephedrine hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Sildec
Look-Alike Drug Names
There is limited information regarding Brompheniramine maleate and Pseudoephedrine hydrochloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.