Skeletal formula
|
WikiDoc Resources for Skeletal formula |
|
Articles |
|---|
|
Most recent articles on Skeletal formula Most cited articles on Skeletal formula |
|
Media |
|
Powerpoint slides on Skeletal formula |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Skeletal formula at Clinical Trials.gov Trial results on Skeletal formula Clinical Trials on Skeletal formula at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Skeletal formula NICE Guidance on Skeletal formula
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Skeletal formula Discussion groups on Skeletal formula Patient Handouts on Skeletal formula Directions to Hospitals Treating Skeletal formula Risk calculators and risk factors for Skeletal formula
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Skeletal formula |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
The skeletal formula of an organic compound is a shorthand representation of its molecular structure. Skeletal formulae are ubiquitous in organic chemistry because they show complicated structures clearly and they are quick and simple to draw.
Carbon skeleton
The term skeletal refers to the carbon skeleton of an organic compound - that is, the chains, branches and/or rings of carbon atoms that form the basis of the structure of an organic molecule. The skeleton may have other atoms or groups of atoms bonded to its carbons. Hydrogen is the most common non-carbon atom bonded to carbon and is not explicitly drawn. Other atoms are known as heteroatoms and groups of atoms are called functional groups, as they give the molecule a function. Heteroatoms and functional groups are known collectively as substituents, as they are considered to be a substitute for the hydrogen atom that would be present in the parent hydrocarbon of organic compound in question.
Implicit carbon and hydrogen atoms
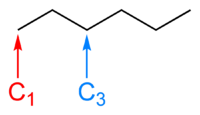
In standard chemical formulae, carbon atoms are represented by the symbol C and hydrogen atoms by the symbol H. In skeletal formulae, the location of carbon atoms, and hydrogen atoms bonded to carbon, are not denoted by the symbols C and H, but are implicit. Carbon atoms are implied to exist at each vertex. Carbon atoms are assumed to have four covalent bonds to them, so the number of hydrogen atoms attached to a particular carbon atom can be deduced by subtracting from four the number of bonds drawn to that carbon.
For example, in the image to the right, the skeletal formula of hexane is shown. The carbon atom labelled C1 has only one bond shown to it, so there must also be three hydrogens bonded to it, in order to make its total number of bonds four. The carbon atom labelled C2 has two bonds to it and is therefore also bonded to two hydrogen atoms.
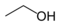
Any hydrogen atoms bonded to non-carbon atoms are drawn explicitly. In ethanol, C2H5OH, for instance, the hydrogen atom bonded to oxygen is denoted by the symbol H, whereas the hydrogens bonded to carbon atoms are not shown directly. Lines representing heteroatom-hydrogen bonds are usually omitted for clarity and compactness, so a functional group like the hydroxyl group is most often written −OH instead of −O−H. These bonds are sometimes drawn out in full in order to accentuate their presence in reaction mechanisms.
Explicit heteroatoms
All atoms that are not carbon or hydrogen are signified by their chemical symbol, i.e. Cl for chlorine, O for oxygen and Na for sodium. These atoms are commonly known as heteroatoms in the context of organic chemistry.
Pseudoelement symbols
There are also symbols that appear to be chemical element symbols, but represent certain very common substituents or indicate an unspecified member of a group of elements. These are known as pseudoelement symbols. The most widely used symbol is Ph, which represents the phenyl group. A list of pseudoelement symbols is shown below:
Elements
Alkyl groups
- R for any alkyl group or even any substituent at all
- Me for the methyl group
- Et for the ethyl group
- n-Pr for the propyl group
- i-Pr for the isopropyl group
- Bu for the butyl group
- i-Bu for the isobutyl group
- s-Bu for the secondary butyl group
- t-Bu for the tertiary butyl group
- Pn for the pentyl group
- Hx for the hexyl group
- Hp for the heptyl group
- Cy for the cyclohexyl group
Aromatic substituents
- Ar for any aromatic substituent
- Bn for the benzyl group
- Bz for the benzoyl group
- Ph for the phenyl group
- Tol for the tolyl group
- Xy for the xylyl group
Functional groups
- Ac for the acetyl group (Ac is also the symbol for the element actinium. However, actinium is rarely encountered in organic chemistry, so the use of Ac to represent the acetyl group never causes confusion)
Leaving groups
See the article leaving group for further information
Multiple bonds
Two atoms can be bonded by sharing more than one pair of electrons. The common bonds to carbon are single, double and triple bonds. Single bonds are most common and are represented by a single, solid line between two atoms in a skeletal formula. Double bonds are denoted by two parallel lines, and triple bonds are shown by three parallel lines.
In more advanced theories of bonding, non-integer values of bond order exist. In these cases, a combination of solid and dashed lines indicate the integer and non-integer parts of the bond order, respectively.
-
Hex-3-ene has an internal carbon-carbon double bond -
Hex-1-ene has a terminal double bond -
Hex-3-yne has an internal carbon-carbon triple bond -
Hex-1-yne has a terminal carbon-carbon triple bond
N.B. in the gallery above, double bonds have been shown in red and triple bonds in blue. This was added for clarity - multiple bonds are not normally coloured in skeletal formulae.
Benzene rings
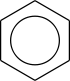
Benzene rings are very common in organic compounds. To represent the delocalization of electrons over the six carbon atoms in the ring, a circle is drawn inside the hexagon of single bonds. This style is very common in introductory organic chemistry texts used in schools.
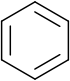
An alternative style that is more common in academia is the Kekulé structure. Although it could be considered inaccurate as it implies three single bonds and three double bonds (benzene would therefore be 1,3,5-cyclohexatriene), all qualified chemists are fully aware of the delocalization in benzene. Kekulé structures are useful for drawing reaction mechanisms clearly.
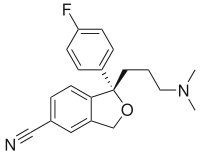
Stereochemistry
Stereochemistry is conveniently denoted in skeletal formulae:
- solid lines represent bonds in the plane of the paper or screen
- wedges represent bonds that point out of the plane of the paper or screen, towards the observer
- dashed lines represent bonds that point into the plane of the paper or screen, away from the observer
- wavy lines represent either unknown stereochemistry or a racemic mixture of the two possible enantiomers
-
2-chloro-2-fluoropentane -
The stereochemical skeletal formula of 2-chloro-2-fluoropentane -
The other possibility for 2-chloro-2-fluoropentane -
The skeletal formula of amphetamine, showing its racemic nature
Hydrogen bonds
Hydrogen bonds are sometimes denoted by dotted or dashed lines
External links
Drawing organic molecules from chemguide.co.uk
