Bergmann degradation
Jump to navigation
Jump to search
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The Bergmann degradation is series of chemical reactions designed to remove a single amino acid from the carboxylic acid end of a peptide.[1][2]
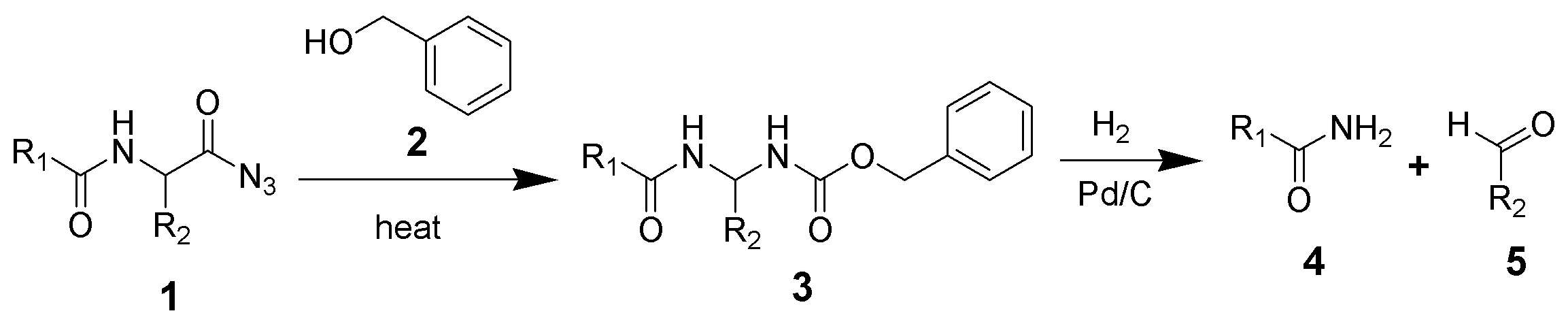
The acyl azide of a peptide (1) undergoes a Curtius rearrangement in the presence of benzyl alcohol (2) to give a benzyl carbamate (3). The Cbz group of intermediate 3 is removed by hydrogenolysis to give an unsubstituteda amide (4) and an aldehyde (5).
References
- ↑ Bergmann, M. Science 1934, 79, 439.
- ↑ Bergmann, M.; Zervas, L. J. Biol. Chem. 1936, 113, 341.
See also