Azelastine (nasal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Azelastine (nasal) is an antihistamine that is FDA approved for the treatment of allergic rhinitis. Common adverse reactions include abnormal taste in mouth, bitter, headache, somnolence, burning sensation in eye, epistaxis, nasal irritation, pharyngitis, rhinitis, sneezing, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Seasonal Allergic Rhinitis
- Dosing information (In adults and adolescents 12 years of age and older)
- Recommended dose: 1 or 2 sprays per nostril twice daily. or 2 sprays per nostril once daily.
Perennial Allergic Rhinitis
- Dosing information (In adults and adolescents 12 years of age and older)
- Recommended dose: 2 sprays per nostril twice daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of azelastine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of azelastine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Seasonal Allergic Rhinitis
- Dosing information
- Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Perennial Allergic Rhinitis
- Dosing information
- Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of azelastine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of azelastine in pediatric patients.
Contraindications
Astelin® Nasal Spray is contraindicated in patients with a known hypersensitivity to azelastine hydrochloride or any of its components.
Warnings
Activities Requiring Mental Alertness
In clinical trials, the occurrence of somnolence has been reported in some patients taking azelastine hydrochloride nasal solution (nasal spray). Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as operating machinery or driving a motor vehicle after administration of azelastine hydrochloride nasal solution (nasal spray). Concurrent use of azelastine hydrochloride nasal solution (nasal spray) with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
Azelastine Hydrochloride Nasal Solution (nasal spray), 0.1%
The safety data described below reflect exposure to azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener in 713 patients 12 years of age and older from 2 clinical trials of 2 weeks to 12 months duration. In a 2-week, double-blind, placebo-controlled, and active-controlled (azelastine hydrochloride nasal solution (nasal spray) without sweetener; azelastine hydrochloride) clinical trial, 285 patients (115 males and 170 females) 12 years of age and older with seasonal allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener one or two sprays per nostril daily. In the 12 month open-label, active-controlled (azelastine hydrochloride nasal solution (nasal spray) without sweetener) clinical trial, 428 patients (207 males and 221 females) 12 years of age and older with perennial allergic rhinitis and/or non allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener two sprays per nostril twice daily. The racial and ethnic distribution for the 2 clinical trials was 82% white, 8% black, 6% Hispanic, 3% Asian, and <1% other.
Adults and Adolescents 12 Years of Age and Older
In the two week clinical trial, 835 patients 12 years of age and older with seasonal allergic rhinitis were treated with one of six treatments: one spray per nostril of either azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener, azelastine hydrochloride nasal solution (nasal spray) without sweetener or placebo twice daily; or 2 sprays per nostril of azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener, azelastine hydrochloride nasal solution (nasal spray) without sweetener, or placebo twice daily. Overall, adverse reactions were more common in the azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener treatment groups (21-28%) than in the placebo groups (16-20%). Overall, less than 1% of patients discontinued due to adverse reactions and withdrawal due to adverse reactions was similar among the treatment groups. Table 1 contains adverse reactions reported with frequencies greater than or equal to 2% and more frequently than placebo in patients treated with azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener in the controlled clinical trial described above.
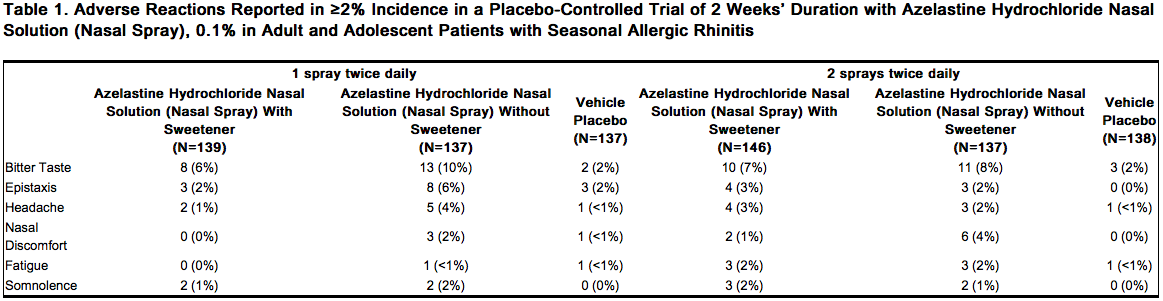
Long-Term (12 Month) Safety Trial
In the 12 month, open-label, active-controlled, long-term safety trial, 862 patients 12 years of age and older with perennial allergic and/or non allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener two sprays per nostril twice daily or azelastine hydrochloride nasal solution (nasal spray) without sweetener two sprays per nostril twice daily. The most frequently reported adverse reactions were headache, bitter taste, epistaxis, and nasopharyngitis and were generally similar between treatment groups. Focused nasal examinations were performed and showed that the incidence of nasal mucosal ulceration in each treatment group was approximately 1% at baseline and approximately 1.5% throughout the 12 month treatment period. In each treatment group, 5-7% of patients had mild epistaxis. No patients had reports of nasal septal perforation or severe epistaxis. Twenty-two patients (5%) treated with azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener and 17 patients (4%) treated with azelastine hydrochloride nasal solution (nasal spray) without sweetener discontinued from the trial due to adverse events.
Azelastine Hydrochloride Nasal Solution (nasal spray), 0.15%
The safety data described below reflect exposure to azelastine hydrochloride nasal solution (nasal spray), 0.15% in 1858 patients (12 years of age and older) with seasonal or perennial allergic rhinitis from 8 clinical trials of 2 weeks to 12 months duration. In 7 double-blind, placebo-controlled clinical trials of 2 to 4 weeks duration, 1544 patients (560 males and 984 females) with seasonal or perennial allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% two sprays per nostril once or twice daily. In the 12 month open-label, active-controlled clinical trial, 466 patients (156 males and 310 females) with perennial allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% two sprays per nostril twice daily. Of these 466 patients, 152 had participated in the 4-week placebo-controlled perennial allergic rhinitis clinical trials. The racial distribution for the 8 clinical trials was 80% white, 13% black, 2% Asian, and 5% other.
Adults and Adolescents 12 Years of Age and Older
In the 7 placebo controlled clinical trials of 2 to 4 week duration, 2343 patients with seasonal allergic rhinitis and 540 patients with perennial allergic rhinitis were treated with two sprays per nostril of either azelastine hydrochloride nasal solution (nasal spray), 0.15% or placebo once or twice daily. Overall, adverse reactions were more common in the azelastine hydrochloride nasal solution (nasal spray), 0.15% treatment groups (16-31%) than in the placebo groups (11-24%). Overall, less than 2% of patients discontinued due to adverse reactions and withdrawal due to adverse reactions was similar among the treatment groups. Table 2 contains adverse reactions reported with frequencies greater than or equal to 2% and more frequently than placebo in patients treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% in the seasonal and perennial allergic rhinitis controlled clinical trials.

In the above trials, somnolence was reported in <1% of patients treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% (11 of 1544) or vehicle placebo (1 of 1339).
Long-Term (12 Month) Safety Trial
In the 12 month, open-label, active-controlled, long-term safety trial, 466 patients (12 years of age and older) with perennial allergic rhinitis were treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% two sprays per nostril twice daily and 237 patients were treated with mometasone nasal spray two sprays per nostril once daily. The most frequently reported adverse reactions (>5%) with azelastine hydrochloride nasal solution (nasal spray), 0.15% were bitter taste, headache, sinusitis, and epistaxis. Focused nasal examinations were performed and no nasal ulcerations or septal perforations were observed. In each treatment group, approximately 3% of patients had mild epistaxis. No patients had reports of severe epistaxis. Fifty-four patients (12%) treated with azelastine hydrochloride nasal solution (nasal spray), 0.15% and 17 patients (7%) treated with mometasone nasal spray discontinued from the trial due to adverse events.
Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Postmarketing Experience
During the post approval use of azelastine hydrochloride nasal solution (nasal spray), 0.1% and 0.15%, the following adverse reactions have been identified. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions reported include: abdominal pain, nasal burning, nausea, sweet taste, and throat irritation. Additionally, the following adverse reactions have been identified during the post approval use of azelastine hydrochloride nasal solution (nasal spray) without sweetener, 0.1% (total daily dose 0.55 mg to 1.1 mg). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions reported include the following: anaphylactoid reaction, application site irritation, atrial fibrillation, blurred vision, chest pain, confusion, dizziness, dyspnea, facial edema, hypertension, involuntary muscle contractions, nervousness, palpitations, paresthesia, parosmia, paroxysmal sneezing, pruritus, rash, disturbance or loss of sense of smell and/or taste, tachycardia, tolerance, urinary retention, and xerophthalmia.
Drug Interactions
Central Nervous System Depressants
Concurrent use of azelastine hydrochloride nasal solution (nasal spray) with alcohol or other central nervous system depressants should be avoided because reductions in alertness and impairment of central nervous system performance may occur.
Erythromycin and Ketoconazole
Interaction studies investigating the cardiac effects, as measured by the corrected QT interval (QTc), of concomitantly administered oral azelastine hydrochloride and erythromycin or ketoconazole were conducted. Oral erythromycin (500 mg three times daily for 7 days) had no effect on azelastine pharmacokinetics or QTc based on analyses of serial electrocardiograms. Ketoconazole (200 mg twice daily for 7 days) interfered with the measurement of azelastine plasma concentrations on the analytic HPLC; however, no effects on QTc were observed.
Cimetidine
Cimetidine (400 mg twice daily) increased the mean Cmax and AUC of orally administered azelastine hydrochloride (4 mg twice daily) by approximately 65%.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled clinical trials in pregnant women. Azelastine hydrochloride has been shown to cause developmental toxicity in mice, rats, and rabbits. Azelastine hydrochloride nasal solution (nasal spray) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects:
In mice, azelastine hydrochloride caused embryo-fetal death, malformations (cleft palate; short or absent tail; fused, absent or branched ribs), delayed ossification, and decreased fetal weight at approximately 170 times the maximum recommended human daily intranasal dose (MRHDID) in adults (on a mg/m2 basis at a maternal oral dose of 68.6 mg/kg/day which also caused maternal toxicity as evidenced by decreased body weight). Neither fetal nor maternal effects occurred in mice at approximately 7 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 3 mg/kg/day).
In rats, azelastine hydrochloride caused malformations (oligo- and brachydactylia), delayed ossification and skeletal variations, in the absence of maternal toxicity, at approximately 150 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 30 mg/kg/day). Azelastine hydrochloride caused embryo-fetal death and decreased fetal weight and severe maternal toxicity at approximately 340 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 68.6 mg/kg/day). Neither fetal nor maternal effects occurred at approximately 15 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 2 mg/kg/day).
In rabbits, azelastine hydrochloride caused abortion, delayed ossification and decreased fetal weight and severe maternal toxicity at approximately 300 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 30 mg/kg/day). Neither fetal nor maternal effects occurred at approximately 3 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 0.3 mg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Azelastine (nasal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Azelastine (nasal) during labor and delivery.
Nursing Mothers
It is not known whether azelastine hydrochloride is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when azelastine hydrochloride nasal solution (nasal spray) is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of azelastine hydrochloride nasal solution (nasal spray) in pediatric patients 6 to 17 years of age have been established. The safety and effectiveness of azelastine hydrochloride nasal solution (nasal spray) in pediatric patients below 6 years of age have not been established. Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Geriatic Use
Clinical trials of azelastine hydrochloride nasal solution (nasal spray) did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Azelastine (nasal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Azelastine (nasal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Azelastine (nasal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Azelastine (nasal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Azelastine (nasal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Azelastine (nasal) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Azelastine (nasal) Administration in the drug label.
Monitoring
There is limited information regarding Azelastine (nasal) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Azelastine (nasal) and IV administrations.
Overdosage
There have been no reported overdosages with azelastine hydrochloride nasal solution (nasal spray). Acute overdosage by adults with this dosage form is unlikely to result in clinically significant adverse events, other than increased somnolence, since one 30-mL bottle of azelastine hydrochloride nasal solution (nasal spray), 0.15% contains up to 45 mg of azelastine hydrochloride. Clinical trials in adults with single doses of the oral formulation of azelastine hydrochloride (up to 16 mg) have not resulted in increased incidence of serious adverse events. General supportive measures should be employed if overdosage occurs. There is no known antidote to azelastine hydrochloride nasal solution (nasal spray). Oral ingestion of antihistamines has the potential to cause serious adverse effects in children. Accordingly, azelastine hydrochloride nasal solution (nasal spray) should be kept out of the reach of children.
Pharmacology
| |
Azelastine (nasal)
| |
| Systematic (IUPAC) name | |
| 4-[(4-chlorophenyl)methyl]-2- (1-methylazepan-4-yl)-phthalazin-1-one | |
| Identifiers | |
| CAS number | |
| ATC code | R01 R06AX19 (WHO), S01GX07 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 381.898 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 40% (intranasal) |
| Metabolism | ? |
| Half life | 22 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | intranasal, ocular |
Mechanism of Action
Azelastine hydrochloride, a phthalazinone derivative, exhibits histamine H1-receptor antagonist activity in isolated tissues, animal models, and humans. Azelastine hydrochloride nasal solution (nasal spray) is administered as a racemic mixture with no difference in pharmacologic activity noted between the enantiomers in in vitro studies. The major metabolite, desmethylazelastine, also possesses H1-receptor antagonist activity.
Structure
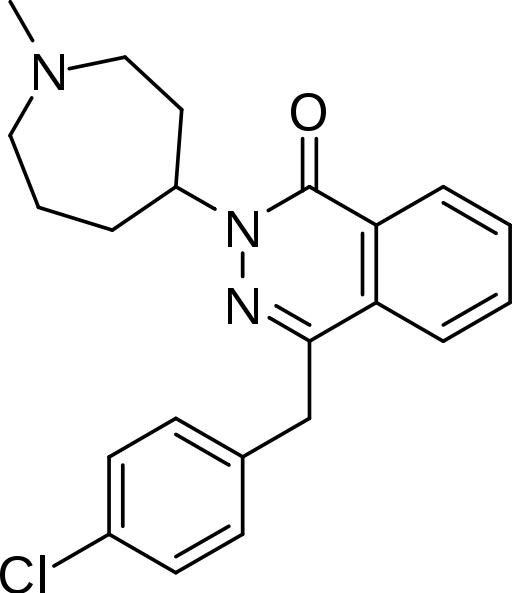
Azelastine hydrochloride nasal solution (nasal spray), 0.15%, 205.5 micrograms (mcg), is formulated as a metered-spray solution for intranasal administration. Azelastine hydrochloride occurs as a white or almost white crystalline powder with a bitter taste. It has a molecular weight of 418.37. It is sparingly soluble in water and soluble in ethanol and in methylene chloride. It has a melting point of about 225°C and the pH of a saturated solution is between 5.0 and 5.4. Its chemical name is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride. Its molecular formula is C22H24ClN3O•HCl with the following chemical structure:

Azelastine hydrochloride nasal solution (nasal spray), 0.15% contains 0.15% azelastine hydrochloride in an isotonic aqueous solution containing benzalkonium chloride (125 mcg/mL), edetate disodium dihydrate, hypromellose, purified water, sodium citrate (dihydrate), sorbitol and sucralose (pH 6.4). After priming, each metered spray delivers a 0.137 mL mean volume containing 205.5 mcg of azelastine hydrochloride (equivalent to 187.6 mcg of azelastine base). The 30-mL (net weight 30 gm of solution) bottle provides 200 metered sprays.
Pharmacodynamics
Cardiac Effects In a placebo-controlled trial (95 patients with allergic rhinitis), there was no evidence of an effect of azelastine hydrochloride nasal spray (2 sprays per nostril twice daily for 56 days) on cardiac repolarization as represented by the corrected QT interval (QTc) of the electrocardiogram. Following multiple dose oral administration of azelastine 4 mg or 8 mg twice daily, the mean change in QTc was 7.2 msec and 3.6 msec, respectively. Interaction studies investigating the cardiac repolarization effects of concomitantly administered oral azelastine hydrochloride and erythromycin or ketoconazole were conducted. Oral erythromycin had no effect on azelastine pharmacokinetics or QTc based on analysis of serial electrocardiograms. Ketoconazole interfered with the measurement of azelastine plasma levels; however, no effects on QTc were observed.
Pharmacokinetics
Absorption
After intranasal administration of 2 sprays per nostril (548 mcg total dose) of azelastine hydrochloride nasal solution (nasal spray), 0.1%, the mean azelastine peak plasma concentration (Cmax) is 200 pg/mL, the mean extent of systemic exposure (AUC) is 5122 pg•hr/mL and the median time to reach Cmax (tmax) is 3 hours. After intranasal administration of 2 sprays per nostril (822 mcg total dose) of azelastine hydrochloride nasal solution (nasal spray), 0.15%, the mean azelastine peak plasma concentration (Cmax) is 409 pg/mL, the mean extent of systemic exposure (AUC) is 9312 pg•hr/mL and the median time to reach Cmax (tmax) is 4 hours. The systemic bioavailability of azelastine hydrochloride is approximately 40% after intranasal administration.
Distribution
Based on intravenous and oral administration, the steady-state volume of distribution of azelastine is 14.5 L/kg. In vitro studies with human plasma indicate that the plasma protein binding of azelastine and its metabolite, desmethylazelastine, are approximately 88% and 97%, respectively.
Metabolism
Azelastine is oxidatively metabolized to the principal active metabolite, desmethylazelastine, by the cytochrome P450 enzyme system. The specific P450 isoforms responsible for the biotransformation of azelastine have not been identified. After a single-dose, intranasal administration of azelastine hydrochloride nasal solution (nasal spray), 0.1% (548 mcg total dose), the mean desmethylazelastine Cmax is 23 pg/mL, the AUC is 2131 pg•hr/mL and the median tmax is 24 hours. After a single-dose, intranasal administration of azelastine hydrochloride nasal solution (nasal spray), 0.15% (822 mcg total dose), the mean desmethylazelastine Cmax is 38 pg/mL, the AUC is 3824 pg•hr/mL and the median tmax is 24 hours. After intranasal dosing of azelastine to steady-state, plasma concentrations of desmethylazelastine range from 20-50% of azelastine concentrations.
Elimination
Following intranasal administration of azelastine hydrochloride nasal solution (nasal spray), 0.1%, the elimination half-life of azelastine is 22 hours while that of desmethylazelastine is 52 hours. Following intranasal administration of azelastine hydrochloride nasal solution (nasal spray), 0.15%, the elimination half-life of azelastine is 25 hours while that of desmethylazelastine is 57 hours. Approximately 75% of an oral dose of radiolabeled azelastine hydrochloride was excreted in the feces with less than 10% as unchanged azelastine.
Special Populations
Hepatic Impairment: Following oral administration, pharmacokinetic parameters were not influenced by hepatic impairment. Renal Impairment: Based on oral, single-dose studies, renal insufficiency (creatinine clearance <50 mL/min) resulted in a 70-75% higher Cmax and AUC compared to healthy subjects. Time to maximum concentration was unchanged. Age: Following oral administration, pharmacokinetic parameters were not influenced by age. Gender: Following oral administration, pharmacokinetic parameters were not influenced by gender. Race: The effect of race has not been evaluated.
Drug-Drug Interactions
Erythromycin: Co-administration of orally administered azelastine (4 mg twice daily) with erythromycin (500 mg three times daily for 7 days) resulted in Cmax of 5.36 ± 2.6 ng/mL and AUC of 49.7 ± 24 ng•h/mL for azelastine, whereas, administration of azelastine alone resulted in Cmax of 5.57 ± 2.7 ng/mL and AUC of 48.4 ± 24 ng•h/mL for azelastine.
Cimetidine and Ranitidine: In a multiple-dose, steady-state drug interaction trial in healthy subjects, cimetidine (400 mg twice daily) increased orally administered mean azelastine (4 mg twice daily) concentrations by approximately 65%. Co-administration of orally administered azelastine (4 mg twice daily) with ranitidine hydrochloride (150 mg twice daily) resulted in Cmax of 8.89 ± 3.28 ng/mL and AUC of 88.22 ± 40.43 ng•h/mL for azelastine, whereas, administration of azelastine alone resulted in Cmax of 7.83 ± 4.06 ng/mL and AUC of 80.09 ± 43.55 ng•h/mL for azelastine.
Theophylline: No significant pharmacokinetic interaction was observed with the co-administration of an oral 4 mg dose of azelastine hydrochloride twice daily and theophylline 300 mg or 400 mg twice daily.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies in rats and mice, azelastine hydrochloride did not show evidence of carcinogenicity at oral doses up to 30 mg/kg and 25 mg/kg, respectively. These doses were approximately 150 and 60 times the maximum recommended human daily intranasal dose [MRHDID] on a mg/m2 basis. Azelastine hydrochloride showed no genotoxic effects in the Ames test, DNA repair test, mouse lymphoma forward mutation assay, mouse micronucleus test, or chromosomal aberration test in rat bone marrow. Reproduction and fertility studies in rats showed no effects on male or female fertility at oral doses up to 30 mg/kg (approximately 150 times the MRHDID in adults on a mg/m2 basis). At 68.6 mg/kg (approximately 340 times the MRHDID on a mg/m2 basis), the duration of estrous cycles was prolonged and copulatory activity and the number of pregnancies were decreased. The numbers of corpora lutea and implantations were decreased; however, pre-implantation loss was not increased.
Clinical Studies
Seasonal Allergic Rhinitis
Azelastine hydrochloride nasal solution (nasal spray), 0.1%
The efficacy and safety of azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener was evaluated in a 2-week, randomized, multicenter, double-blind, placebo-controlled clinical trial including 834 adult and adolescent patients 12 years of age and older with symptoms of seasonal allergic rhinitis. The population was 12 to 83 years of age (60% female, 40% male; 69% white, 16% black, 12% Hispanic, 2% Asian, 1% other). Patients were randomized to one of six treatment groups: 1 spray per nostril of either azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener, azelastine hydrochloride nasal solution (nasal spray) without sweetener or vehicle placebo twice daily; or 2 sprays per nostril of azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener, azelastine hydrochloride nasal solution (nasal spray) without sweetener or vehicle placebo twice daily. Assessment of efficacy was based on the 12-hour reflective total nasal symptom score (rTNSS) assessed daily in the morning and evening, in addition to the instantaneous total nasal symptom score (iTNSS) and other supportive secondary efficacy variables. TNSS is calculated as the sum of the patients’ scoring of the four individual nasal symptoms (rhinorrhea, nasal congestion, sneezing, and nasal itching) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe). The rTNSS required patients to record symptom severity over the previous 12 hours. For the primary efficacy endpoint, the mean change from baseline rTNSS, morning (AM) and evening (PM) rTNSS scores were summed for each day (maximum score of 24) and then averaged over the 2 weeks. The iTNSS, recorded immediately prior to the next dose, were assessed as an indication of whether the effect was maintained over the dosing interval. In this trial, azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener two sprays twice a day demonstrated a greater decrease in rTNSS and iTNSS than placebo and the difference was statistically significant. The trial results are presented in Table 3 (Trial 1). The efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener one spray per nostril twice daily for seasonal allergic rhinitis is supported by two, 2-week, placebo-controlled clinical trials with azelastine hydrochloride nasal solution (nasal spray) without sweetener in 413 patients with seasonal allergic rhinitis. In these trials, efficacy was assessed using the TNSS (described above). Azelastine hydrochloride nasal solution (nasal spray) without sweetener demonstrated a greater decrease from baseline in the summed AM and PM rTNSS compared with placebo and the difference was statistically significant.
Azelastine hydrochloride nasal solution (nasal spray), 0.15%
The efficacy and safety of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener in seasonal allergic rhinitis was evaluated in five randomized, multicenter, double-blind, placebo-controlled clinical trials in 2499 adult and adolescent patients 12 years and older with symptoms of seasonal allergic rhinitis (Trials 2, 3, 4, 5 and 6). The population of the trials was 12 to 83 years of age (64% female, 36% male; 81% white, 12% black, <2% Asian, 5% other; 23% Hispanic, 77% non-Hispanic). Assessment of efficacy was based on the rTNSS, iTNSS as described above, and other supportive secondary efficacy variables. The primary efficacy endpoint was the mean change from baseline in rTNSS over 2 weeks. Two 2-week seasonal allergic rhinitis trials evaluated the efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener dosed at 2 sprays twice daily. The first trial (Trial 2) compared the efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener and azelastine hydrochloride nasal solution (nasal spray) without sweetener to vehicle placebo. The other trial (Trial 3) compared the efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener and azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener to vehicle placebo. In these two trials, azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener demonstrated greater decreases in rTNSS than placebo and the differences were statistically significant (Table 3). Three 2-week seasonal allergic rhinitis trials evaluated the efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener dosed at 2 sprays once daily compared to the vehicle placebo. Trial 4 demonstrated a greater decrease in rTNSS than placebo and the difference was statistically significant (Table 3). Trial 5 and Trial 6 were conducted in patients with Texas mountain cedar allergy. In Trial 5 and Trial 6, azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener demonstrated a greater decrease in rTNSS than placebo and the differences were statistically significant (Trials 5 and 6; Table 3). Instantaneous TNSS results for the once daily dosing regimen of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener are shown in Table 4. In Trials 5 and 6, azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener demonstrated a greater decrease in iTNSS than placebo and the differences were statistically significant.


Azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener at a dose of 1 spray twice daily was not studied. The azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener 1 spray twice daily dosing regimen is supported by previous findings of efficacy for azelastine hydrochloride nasal solution (nasal spray) without sweetener and a favorable comparison of azelastine hydrochloride nasal solution (nasal spray), 0.15% with sweetener to azelastine hydrochloride nasal solution (nasal spray), 0.1% without sweetener and azelastine hydrochloride nasal solution (nasal spray), 0.1% with sweetener (Table 3). Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Perennial Allergic Rhinitis
Azelastine hydrochloride nasal solution (nasal spray), 0.15% The efficacy and safety of azelastine hydrochloride nasal solution (nasal spray), 0.15% in perennial allergic rhinitis was evaluated in one randomized, multicenter, double-blind, placebo-controlled clinical trial in 578 adult and adolescent patients 12 years and older with symptoms of perennial allergic rhinitis. The population of the trial was 12 to 84 years of age (68% female, 32% male; 85% white, 11% black, 1% Asian, 3% other; 17% Hispanic, 83% non-Hispanic). Assessment of efficacy was based on the 12-hour reflective total nasal symptom score (rTNSS) assessed daily in the morning and evening, the instantaneous total nasal symptom score (iTNSS), and other supportive secondary efficacy variables. The primary efficacy endpoint was the mean change from baseline rTNSS over 4 weeks. The one 4-week perennial allergic rhinitis trial evaluated the efficacy of azelastine hydrochloride nasal solution (nasal spray), 0.15%, azelastine hydrochloride nasal solution (nasal spray), 0.1% and vehicle placebo dosed at 2 sprays per nostril twice daily. In this trial, azelastine hydrochloride nasal solution (nasal spray), 0.15% demonstrated a greater decrease in rTNSS than placebo and the difference was statistically significant (Table 5).

Pediatric use information for patients ages 6 to 11 years of age for treatment of allergic rhinitis, including seasonal and perennial allergic rhinitis is approved for Meda Pharmaceuticals’ azelastine hydrochloride nasal spray product. However, due to Meda Pharmaceuticals’ marketing exclusivity rights, this drug product is not labeled with that pediatric information.
How Supplied
Azelastine hydrochloride nasal solution (Nasal Spray), 0.15% is supplied as a 30 mL package (NDC 60505-0848-5) delivering 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit. The spray pump unit consists of a nasal spray pump fitted with a safety clip and a plastic dust cover. The net content of the bottle is 30 mL (net weight 30 gm of solution). The 30-mL bottle contains 45 mg (1.5 mg/mL) of azelastine hydrochloride. After priming, each spray delivers a fine mist containing a mean volume of 0.137 mL solution containing 205.5 mcg of azelastine hydrochloride. The correct amount of medication in each spray cannot be assured before the initial priming and after 200 sprays have been used, even though the bottle is not completely empty. The bottle should be discarded after 200 sprays have been used. Azelastine hydrochloride nasal Solution (Nasal Spray), 0.15% should not be used after the expiration date “EXP” printed on the medicine label and carton.
Storage
Store upright at 20° to 25°C (68° to 77°F) [see USP controlled room temperature]. Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Azelastine (nasal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Azelastine (nasal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
Concurrent use of azelastine hydrochloride nasal solution (nasal spray) with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
Brand Names
- Astelin
- Astelin Ready-Spray
- Optivar
- Astepro
Look-Alike Drug Names
Astelin - Avastin[1]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Azelastine (nasal) |Label Name=Azelastine_label_01.jpg
}}
{{#subobject:
|Label Page=Azelastine (nasal) |Label Name=Azelastine_label_02.jpg
}}
{{#subobject:
|Label Page=Azelastine (nasal) |Label Name=Azelastine_panel_01.png
}}