Asciminib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Asciminib is a kinase inhibitor that is FDA approved for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia with disease that meets certain criteria. Common adverse reactions include include fatigue, nausea, diarrhea, rash, musculoskeletal pain, and upper respiratory tract infections.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
For Patients with Ph+ CML-CP, previously treated with two or more TKIs should either take:
- 80 mg taken once a day orally
- 40 mg twice a day in 12 hour intervals orally
For Patients with Ph+ CML-CP with the T315I mutation should take:
- 200 mg twice a day in 12 hour intervals orally
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Asciminib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Asciminib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Asciminib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Asciminib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Asciminib in pediatric patients.
Contraindications
There are no contraindications associated with Asciminib.
Warnings
Myelosuppression
- Studies conducted show evidence of thrombocytopenia, neutropenia, and anemia.
- Thrombocytopenia was found in 28% of patients taking Asciminib. Of those patients, Grade 3 thrombocytopenia occurred in 7% of patients and Grade 4 thrombocytopenia occurred in 12% of patients.
- Neutropenia was found in 19% of patients taking Asciminib. Of those patients, Grade 3 neutropenia occurred in 8% of patients and Grade 4 neutropenia occurred in 8% of patients.
- Anemia was found in 13% of patients taking Asciminib. Of those patients, Grade 3 anemia occurred in 5% of patients.
- To monitor symptoms, blood counts should be conducted every 2 weeks of initial start to monthly checks after 3 months of treatment. Alter dosage or permanently discontinue Asciminib based on severity of symptoms.
Pancreatic Toxicity
- Serum lipase and amylase should be monitored monthly in patients using Asciminib and frequently in patients that have a history of pancreatitis. *Studies showed pancreatitis in 2.5% of patients with Grade 3 pancreatitis occurring in 1.1% of patients.
- Increase in lipase and amylase occurred in 21% of patients. Of those patients, Grade 3 pancreatic enzyme elevation occurred in 10% of patients and Grade 4 pancreatic enzyme elevation occurred in 2.2% of patients.
- Alter or discontinue the use of Asciminib when levels of serum lipase and amylase change.
Hypertension
- Hypertension should be monitored and treated appropriately. Studies conducted show that hypertension occurred in 19% patients. Of those patients, Grade 3 hypertension was found in 9% of patients and Grade 4 hypertension was found in 0.3% of patients.
- Advise patients who have symptoms of Grade 3 and higher hypertensions to either reduce, temporarily withold, or permanently discontinue the use of Asciminib depending on the severity of the symptoms.
Hypersensitivity
- Hypersensitivity including rash, edema, and bronchospasm have been reported in patients using Asciminib. Studies conducted show that hypersensitivity occurred in 32% patients. Of those patients, Grade 3 or Grade 4 hypersensitivity was found in 1.7% of patients.
- Patients with symptoms of Grade 3 and higher hypersensitivity should either reduce, temporarily withold, or permanently discontinue the use of Asciminib depending on the severity of the symptoms.
Cardiovascular Toxicity
- Cardiovascular toxicity including ischemic cardiac, arterial thrombotic and embolic conditions have been reported in patients using Asciminib. Studies conducted show that cardiovascular toxicity occurred in 13% patients while 2.2% of patients had cardiac failure. Of those patients, Grade 3 cardiovascular toxicity occurred in 3.4% of patients and Grade 3 cardiac failure occurred in 1.1% of patients. The study also showed Grade 4 cardiovascular toxicity occurred in 0.6% of patients.
- Monitor patients regularly who have a history of cardiovascular risks when taking Asciminib. Patients with symptoms of Grade 3 and higher cardiovascular toxicity should either reduce, temporarily withold, or permanently discontinue the use of Asciminib depending on the severity of the symptoms.
Embryo-Fetal Toxicity
- Based on animal data, Asciminib potentially can cause harm to females fetus during pregnancy. Animal studies show mortality and malformations in rats and rabbits occurred during organogenesis.
- Advise females about potential risks to a fetus when taking Asciminib. *Advise females of reproductive potential to use effective contraception during treatment with Asciminib and for at least 1 week after the last dose.
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Asciminib has been evaluated for safety in 356 patients who were observed in one of two clinical trials with one trial including patients with Ph+ CML-CP, previously treated with two or more TKIs and the other trial including patients with Ph+ CML-CP with the T315I mutation. Both trials are discussed below and had a median duration of 89 weeks.
Patients with Ph+ CML-CP, previously treated with two or more TKIs
- 15% of patients taking Asciminib received adverse reactions such as pyrexia (1.9%), cardiac failure congestive (1.3%), thrombocytopenia (1.3%), and urinary tract infection (1.3%).
- 7% of patients who had adverse reactions had to permanently discontinue Asciminib with reactions including thrombocytopenia (3.2%) and neutropenia (2.6%).
- 38% of patients had interruptions in their dosage due to adverse reactions which included thrombocytopenia (19%) and neutropenia (18%).
- Dosage reductions occurred due to adverse reactions in 38% of patients which showed thrombocytopenia (19%) and neutropenia (18%).
- Upper respiratory tract infections and musculoskeletal pain were the most common adverse reactions (≥ 20%) in patients.
Table 3 shows the overall adverse reactions in Patients with Ph+ CML-CP, previously treated with two or more TKIs taking Asciminib compared to Bosutinib.
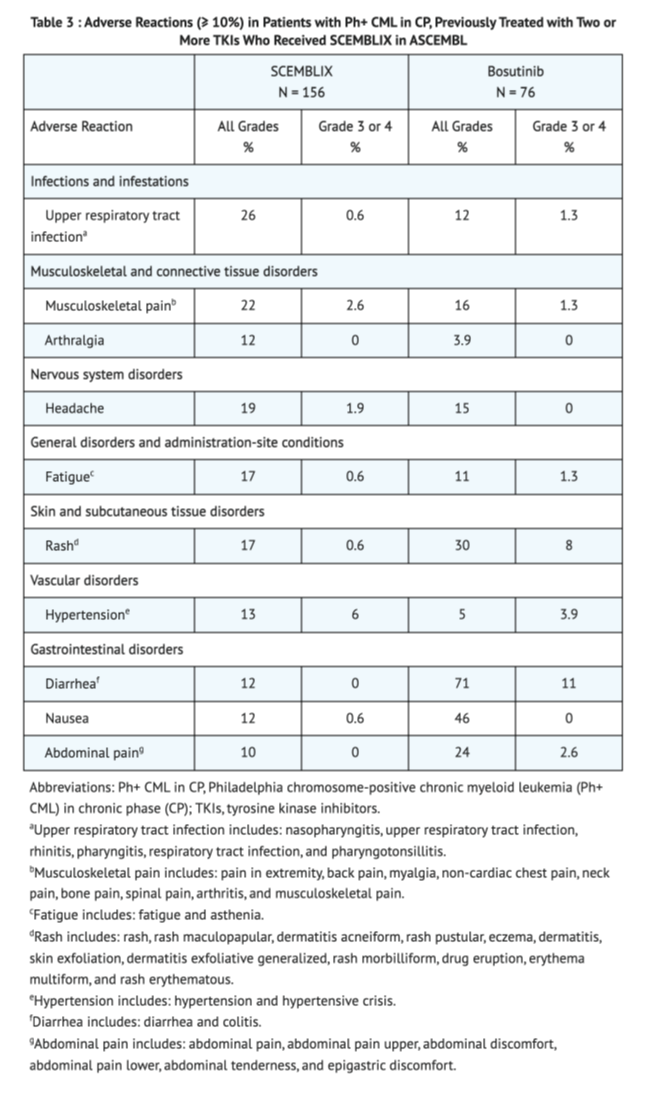
Table 4 shows the overall lab abnormalities in Patients with Ph+ CML-CP, previously treated with two or more TKIs taking Asciminib in ASCEMBL.
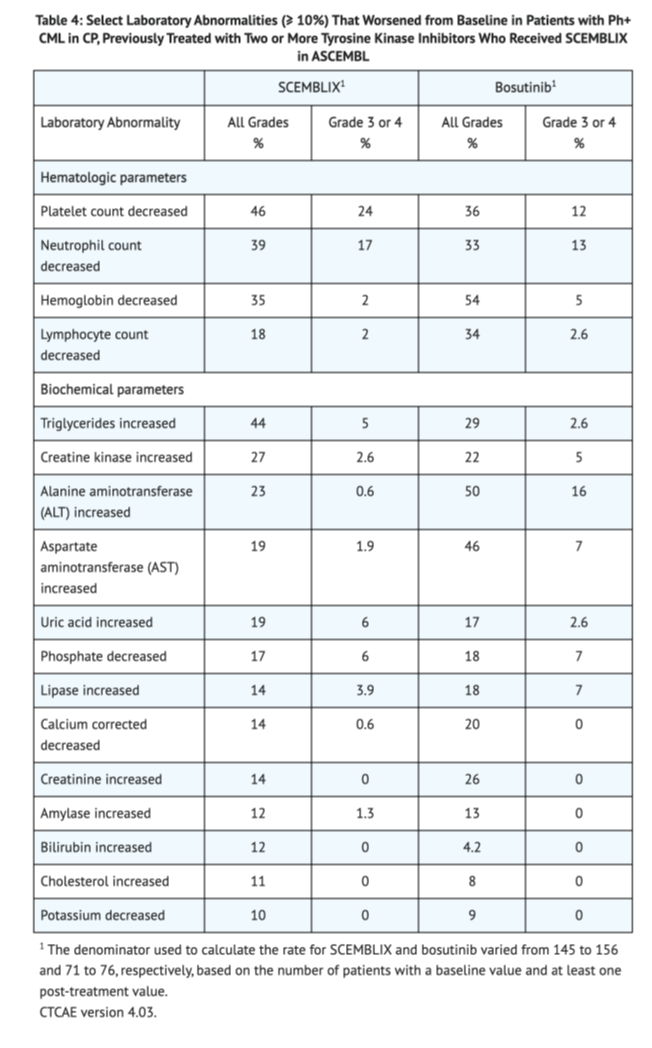
Patients with Ph+ CML-CP with the T315I mutation
- 23% of the patients taking Asciminib displayed adverse reactions such as abdominal pain (4.2%), vomiting (4.2%), pneumonia (4.2%), musculoskeletal pain (2.1%), headache (2.1%), hemorrhage (2.1%), constipation (2.1%), arrhythmia (2.1%), and pleural effusion (2.1%).
- 10% of patients who had adverse reactions had to permanently discontinue Asciminib with reactions including pancreatic enzymes increase (2.1%).
- 31% of patients had interruptions in their dosage due to adverse reactions which included thrombocytopenia (19%) and pancreatic enzymes increase (17%).
- Dosage reductions occurred due to adverse reactions in 23% of patients which showed pancreatic enzymes increase (10%), abdominal pain (4.2%), anemia (2.1%), blood bilirubin increase (2.1%), dizziness (2.1%), fatigue (2.1%), hepatic enzymes increase (2.1%), musculoskeletal pain (2.1%), nausea (2.1%), neutropenia (2.1%), pruritus (2.1%), and thrombocytopenia (2.1%). *Musculoskeletal pain, fatigue, nausea, rash, and diarrhea were the most common adverse reactions (≥ 20%) in patients.
Table 5 shows the overall adverse reactions in Patients with Ph+ CML-CP with the T315I mutation taking Asciminib in X2101.
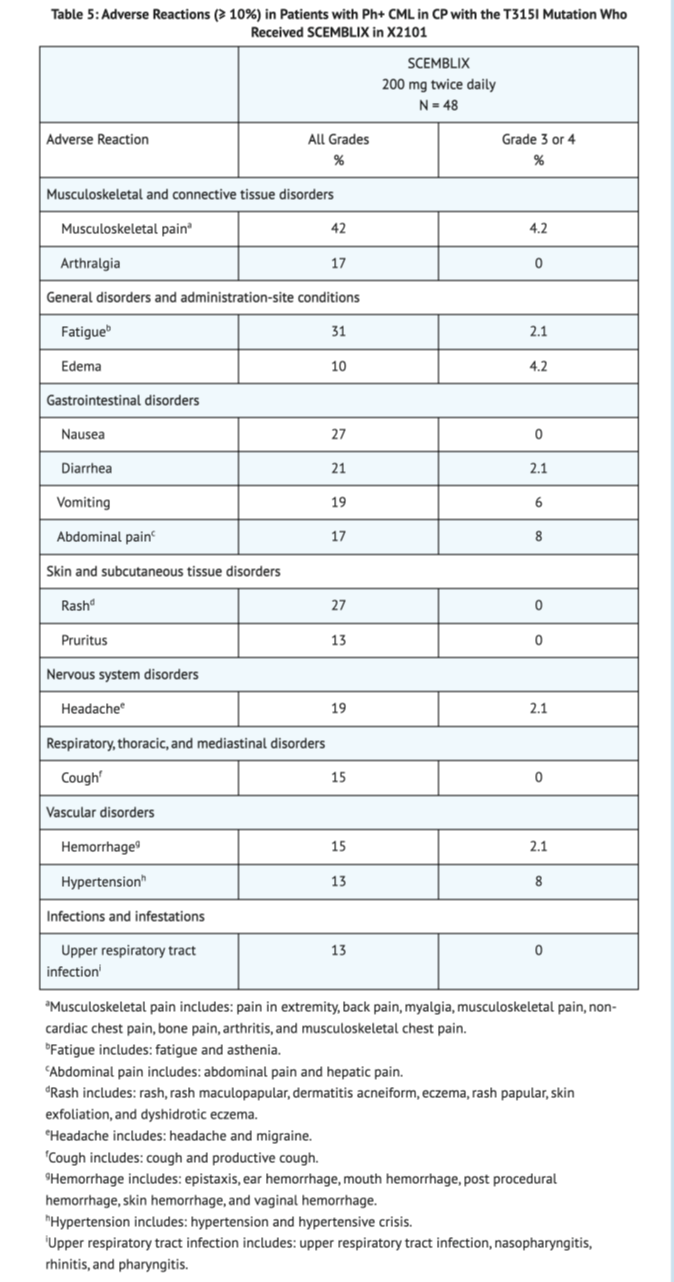
Table 6 shows the overall lab abnormalities in Patients with Ph+ CML-CP with the T315I mutation in X2101
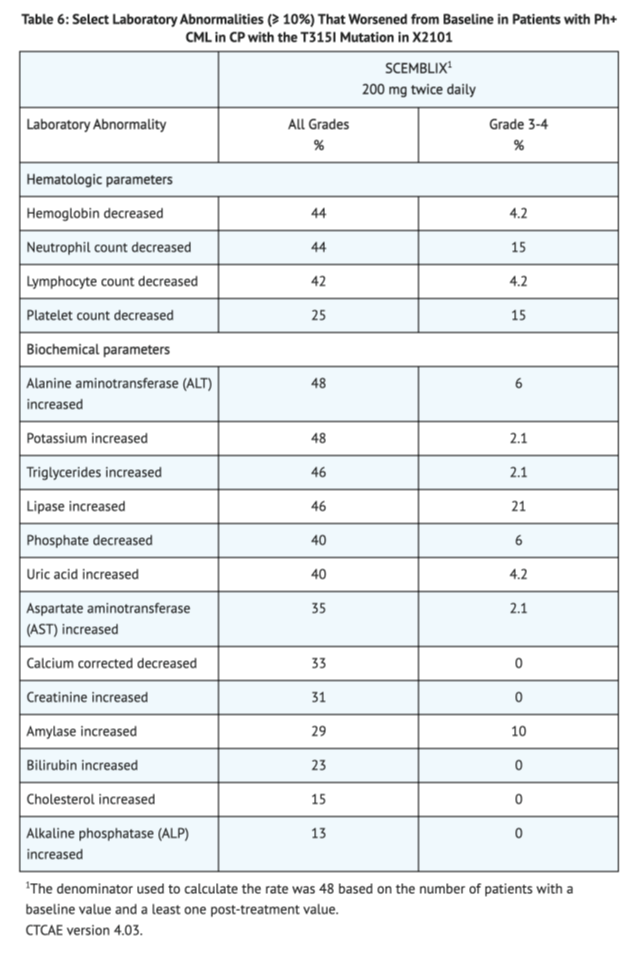
Postmarketing Experience
There is limited information about "Postmarketing Experiance" in the drug label.
Drug Interactions
Strong CYP3A4 Inhibitors
- Concomitant use of these drugs potentially increases the likelihood of adverse effects in patients because Asciminib Cmax and AUC increase with concomitant use.
Itraconazole Oral Use containing Hydroxypropyl-β-cyclodextrin
- Concomitant use of these drugs may decrease Asciminib efficacy in patients as both Asciminib Cmax and AUC decrease with concomitant use.
Certain CYP3A4
- Concomitant use of these drugs potentially increases the likelihood of adverse effects in patients because Asciminib Cmax and AUC of CYP3A4 substrates increase with concomitant use.
- Monitor patients using 80 mg Asciminib with concomitant use of certain CYP3A4 substrates which can potentially lead to adverse reactions in patients.
- Advise patients to avoid the coadministration of 200 mg Asciminib and certain CYP3A4 substrates which can potentially lead to adverse reactions in patients.
CYP2C9 Substrates
- Concomitant use of these drugs potentially increases the likelihood of adverse effects in patients because because Asciminib Cmax and AUC of CYP2C9 substrates increase with concomitant use.
- Advise patients to avoid coadministration of both 80 mg and 200 mg Asciminib with certain CYP2C9 substrates which can potentially lead to adverse reactions in patients.
Certain P-gp Substrates
- Concomitant use of these drugs potentially increases the likelihood of adverse effects in patients because of the increase in plasma membrane concentration of these substrates.
- Monitor patients for potential adverse reactions at all dosages of Asciminib with concomitant use of certain P-gp Substrates.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Reproduction studies done on rats and rabbits at doses up to 600 mg/kg/day and 300 mg/kg/day, respectively, revealed both maternal toxicity at the highest doses and malformations in different areas of the body. In rats, malformations occurred in cleft palate, anasarca (edema), and cardiac abnormalities at 150 mg/kg. In rabbits, studies showed cardiac malformations and decrease in live fetus in females at 50mg/kg. These studies display the potential harms and risks in the embryo of pregnant woman when taking Asciminib.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Asciminib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Asciminib during labor and delivery.
Nursing Mothers
No data has been conducted on nursing in human when taking Asciminib. Based on studies done on rats and rabbits, it is recommended not to lactate when taking Asciminib.
Pediatric Use
Safety and effectiveness in pediatric populations have not been established.
Geriatic Use
Of the total number of subjects in the ASCEMBL clinical studies, around 19% of the patients were 65 years or older in age, and 2.6% were 75 years or older in age. In X2101 that tested patients with T315I mutation, around 33% of the patients were 65 years or older in age, and 8% were 75 years or older in age. No differences among young patients compared to patients 65 years or older in age were found when looking at safety and efficacy of Asciminib. More data is required to assess safety and efficacy between young patients and patients 75 year or older in age.
Gender
There is no FDA guidance on the use of Asciminib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Asciminib with respect to specific racial populations.
Renal Impairment
Patients with mild and severe renal impairment require no change to dosage usage.
Hepatic Impairment
Patients with mild and severe hepatic impairment require no change to dosage usage.
Females of Reproductive Potential and Males
Infertility can be impaired in females based on studies done on rats and rabbits. Asciminib has shown signs of harm in the embryo of pregnant women. There has been no effects recorded in males on their reproductive potential.
Immunocompromised Patients
There is no FDA guidance on the use of Asciminib with respect to immunocompromised populations.
Administration and Monitoring
Administration
- Swallow Asciminib tablets whole.
- Instruct patients to not chew, crush, or break tablets.
- Instruct patients to not eat 2 hours before taking tablet and 1 hour after taking tablet.
- Continue treatment until disease progression or unacceptable toxicity.
- Follow recommended dosage as prescribed by a doctor.
- For pateints that take Asciminib once daily, if a dosage is missed by more than 12 hours, then skip dosage and take next dosage at scheduled time.
- For pateints that take Asciminib twice daily, if a dosage is missed by more than 6 hours, then skip dosage and take next dosage at scheduled time.
Monitoring
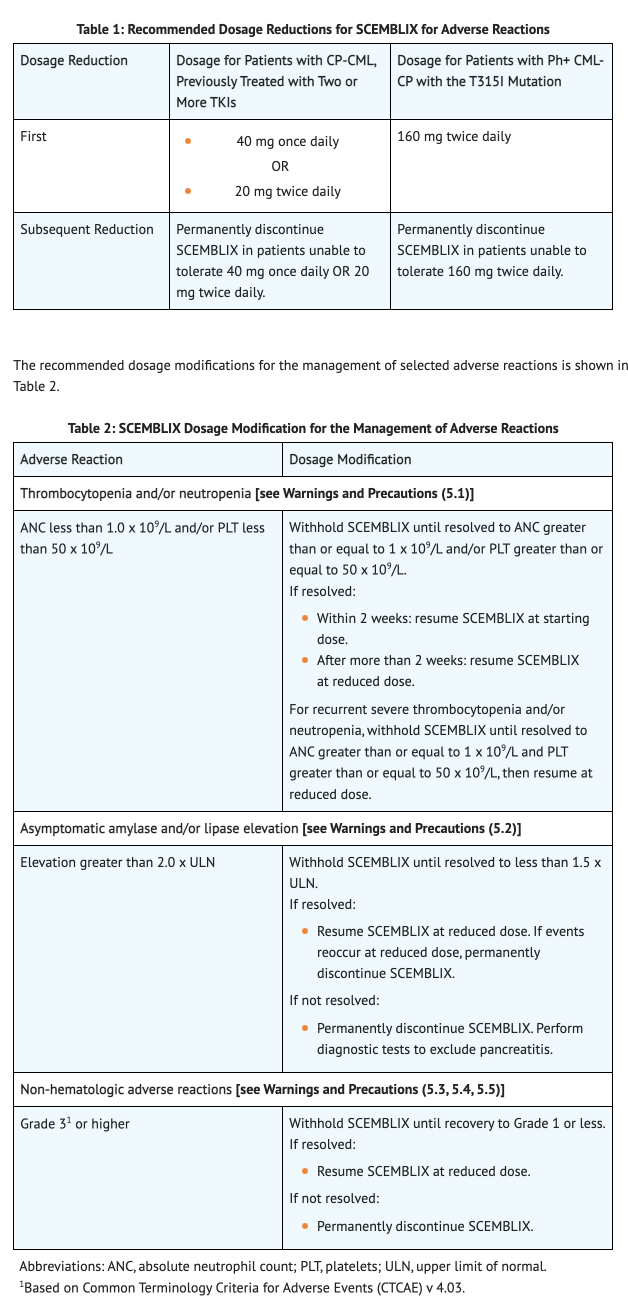
IV Compatibility
There is limited information regarding the compatibility of Asciminib and IV administrations.
Overdosage
There is limited information regarding Asciminib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Asciminib
| |
| Systematic (IUPAC) name | |
| N-4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide;hydrochloride | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 449.80 g/mol |
| Synonyms | ABL001 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 97% |
| Metabolism | ? |
| Half life | ? |
| Excretion | 80% via feces, 11% via urine |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- Asciminib is an ABL/BCR-ABL1 tyrosine kinase inhibitor that has an inhibitory effect on the ABL1 Protein. Kinase activity of the ABL1 protein is inhibited on the BCR-ABL1 fusion protein.
- In vitro, Asciminib also showed inhibitory effects against several mutant forms of BCR-ABL1 which also included the T315I mutation.
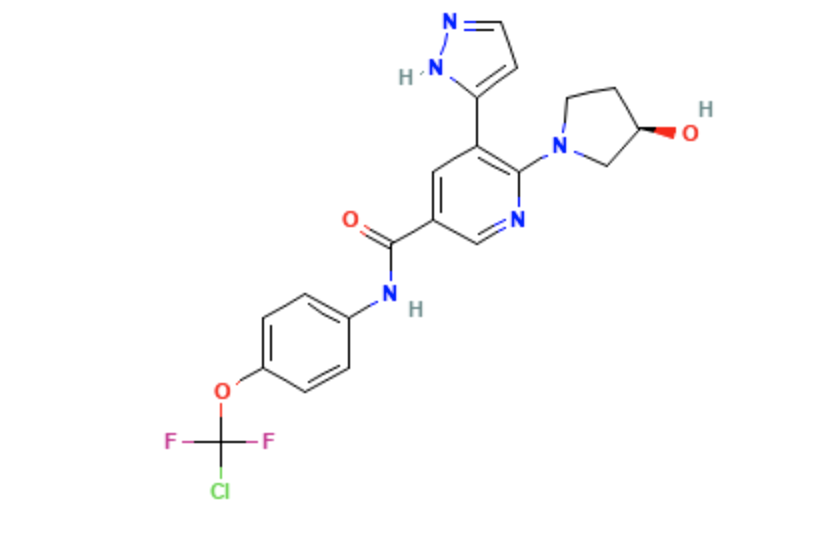
Structure
- Asciminib is a kinase inhibitor for oral administration. It has an empirical formula of C20H18ClF2N5O3 and a molecular weight of 449.8.
- The chemical name is N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide.

Pharmacodynamics
Exposure-Response Relationships
- Lower exposure of asciminib dosages of 10mg to 200mg twice daily at week 24 was associated with a smaller decrease in BCR-ABL1 level and a lower MMR rate.
- Higher exposure of asciminib dosages of 10mg to 280mg twice daily was associated with adverse reactions occurring more frequently.
Cardiac Electrophysiology
- At maximum clincal dosage, no large mean increase in QTc interval is indicated when taking Asciminib.
Pharmacokinetics
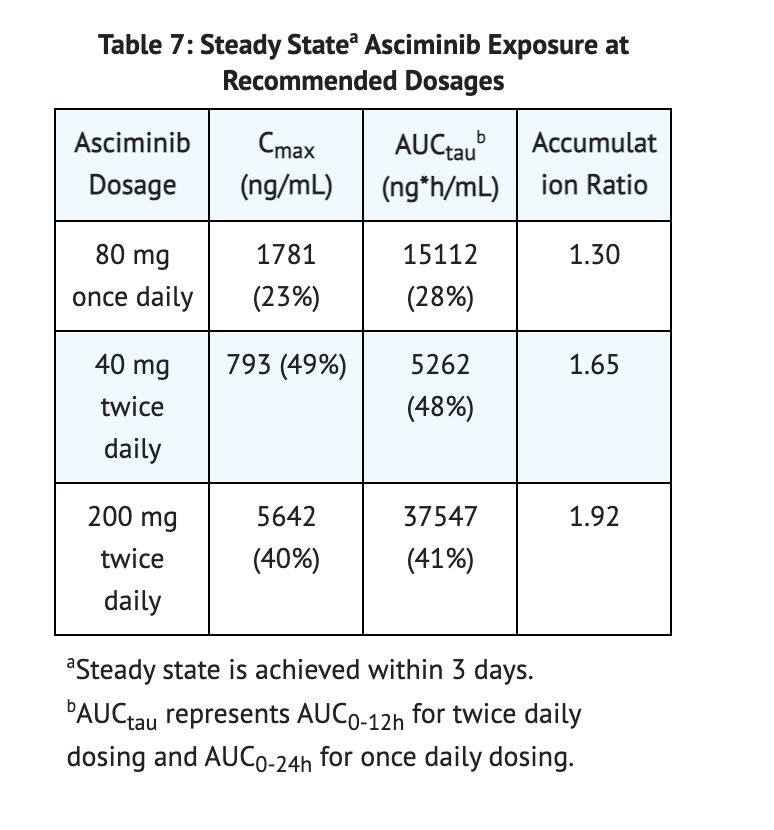
Steady State Exposure
- At 10 mg to 200 mg of Asciminib, AUC and Cmax increased slightly higher in comparison to the dose proportion.
Table 7 shows data conducted on Steady State Asciminib Exposure
Absorption
- Asciminib has a median Tmax of 2.5 hours.
- In high fat meals (1000 calories, 50% fat), AUC decreased by 62% and Cmax decreased by 68%.
- In low fat meals (400 calories, 25% fat), AUC decreased by 30% and Cmax decreased by 35%.
Distribution
- At a steady state, the volume of distribution is 151 L for Asciminib.
- In human plasma protein in vitro, Asciminib is 97% bound.
Elimination
- Total clearance at 40 mg twice daily and 80 mg once daily of Asciminib is 6.7 L/hour.
- Total clearance at 200 mg twice daily of Asciminib is 4.1 L/hour.
- Terminal elimination half-life at 40 mg twice daily and 80 mg once daily of Asciminib is 5.5 hours.
- Terminal elimination half-life at 200 mg twice daily of Asciminib is 9.0 hours.
Metabolism
- UGT2B7-mediated glucuronidation, UGT2B17-mediated glucuronidation, CYP3A4-mediated oxidation metabolizes Asciminib.
Excretion
- In feces after a single 80 mg dosage of Asciminib, 80% of Asciminib was found in which 57% was found unchanged.
- In urine, after a single 80 mg dosage of Asciminib, 11% of Asciminib was found in which 2.5% was found unchanged.
- Biliary Secretion eliminates Asciminib via BCRP.
Specific Populations
- Sex, mild to moderate hepatic impairment, age, race, mild to moderate renal impairment, and body weight showed no significant differences of pharmacokinetics clinically of Asciminib.
Patients with Renal Impairment
- Patients not requiring dialysis who also have eGFR between 13 to < 30 mL/min/1.73 m2 showed increases in both AUCinf (57%) and Cmax (6%) after single 40 mg dose of Asciminib when compared to patients with normal renal function.
- Changes in exposure are not considered clinically meaningful.
Patients with Hepatic Impairment
- Patients with severe hepatic impairment (total bilirubin > 3 times ULN and any AST) showed increases in both AUCinf (33%) and Cmax (4%) after single 40 mg dose of Asciminib when compared to patients with normal hepatic function. *Changes in exposure are not considered clinically meaningful.
Drug Interaction Studies
Drugs that Affect Plasma Concentrations of Asciminib
- Strong CYP3A Inhibitors: Coadministration of clarithromycin (Strong CYP3A Inhibitor) and a single dose of 40 mg of Asciminib showed increases in both AUCinf (36%) and Cmax (19%). Coadministration of itraconazole (Strong CYP3A Inhibitor) and Asciminib showed no significant differences in AUCinf and Cmax clinically.
- Strong CYP3A Inducers: Asciminib in concomitant use of strong CYP3A inducers has not been fully characterized.
- Itraconazole Oral Solution: Coadministration of a single dose of 40 mg of Asciminib and itraconazole oral solution containing hydroxypropyl-β-cyclodextrin showed decreases in both AUCinf (40%) and Cmax (50%). Coadministration of Asciminib and other products containing hydroxypropyl-β-cyclodextrin are not fully characterized.
- Imatinib: Coadministration of imatinib and a single dose 40 mg of Asciminib showed increases in both AUCinf (108%) and Cmax (59%). Changes in exposure are not considered clinically meaningful. Concomitant use of 200 mg of Asciminib twice daily and imatinib are not fully characterized.
- Other Drugs: Coadministration of either quinidine (P-gp inhibitor) or rabeprazole (acid-reducing agent) and Asciminib showed no significant differences clinically.
Drugs That are Affected by Asciminib
- CYP3A4 Substrates: Coadministration of midazolam, CYP3A4 substrate, and a twice daily dosage of 40 mg of Asciminib showed increases in both AUCinf (28%) and Cmax (11%). Coadministration of midazolam and a single daily dosage of 80 mg of Asciminib showed increases in both AUCinf (24%) and Cmax (17%). Coadministration of midazolam and a twice daily dosage of 200 mg of Asciminib showed increases in both AUCinf (88%) and Cmax (58%).
- CYP2C9 Substrates: Coadministration of warfarin, CYP2C9 substrate, and a twice daily dosage of 40 mg of Asciminib showed increases in both AUCinf (41%) and Cmax (8%). Coadministration of warfarin and a single daily dosage of 80 mg of Asciminib showed increases in both AUCinf (52%) and Cmax (4%). Coadministration of midazolam and a twice daily dosage of 200 mg of Asciminib showed increases in both AUCinf (314%) and Cmax (7%).
- CYP2C8 Substrates: Coadministration of repaglinide (substrate of CYP2C8, CYP3A4, and OATP1B) and a twice daily dosage of 40 mg of Asciminib showed increases in both AUCinf (8%) and Cmax (14%). Coadministration of repaglinide and a single daily dosage of 80 mg of Asciminib showed increases in both AUCinf (12%) and Cmax (8%). Coadministration of repaglinide and a twice daily dosage of 200 mg of Asciminib showed increases in both AUCinf (42%) and Cmax (25%). Coadministration of rosiglitazone (substrate of CYP2C8 and CYP2C9) and a twice daily dosage of 40 mg of Asciminib showed increases in both AUCinf (20%) and Cmax (3%). Coadministration of rosiglitazone and a single daily dosage of 80 mg of Asciminib showed increases in both AUCinf (24%) and Cmax (2%). Coadministration of rosiglitazone and a twice daily dosage of 200 mg of Asciminib showed increases in both AUCinf (66%) and Cmax (8%).
- P-gp Substrates: Increases in plasma membrane concentrations and serious toxicities may occur with coadministration of P-gp substrates and Asciminib.
In Vitro Studies
- CYP450 and UGT Enzymes: Plasma concentrations reached at 80 mg twice daily and 200 mg twice daily of Asciminib can reversibly inhibit UGT1A1. At 200 mg of Ascminib twice daily can reversibly inhibit CYP2C19.
- Transporter Systems: OATP1B1, BCRP, OATP1B3, P-gp, and OCT1 are inhibited by Asciminib. The substrate of P-gp and BCRP is Asciminib.
Nonclinical Toxicology
- No research has been conducted on the carcinogenicity when dealing with Asciminib.
- When looking at fertility, studies conducted on male rats have shown evidence of a decrease in sperm count and motility when dosed with 200 mg/kg/day of Asciminib. Studies on female mice showed evidence of decreased living embryos when dosed with 200 mg/kg/day of Asciminib.
- Mutagenicity studies have shown that Asciminib is not genotoxic.
Clinical Studies
Two clinical trials were conducted on Patients with Ph+ CML-CP, previously treated with two or more TKIs and Patients with Ph+ CML-CP with the T315I mutation.
Patients with Ph+ CML-CP, previously treated with two or more TKIs
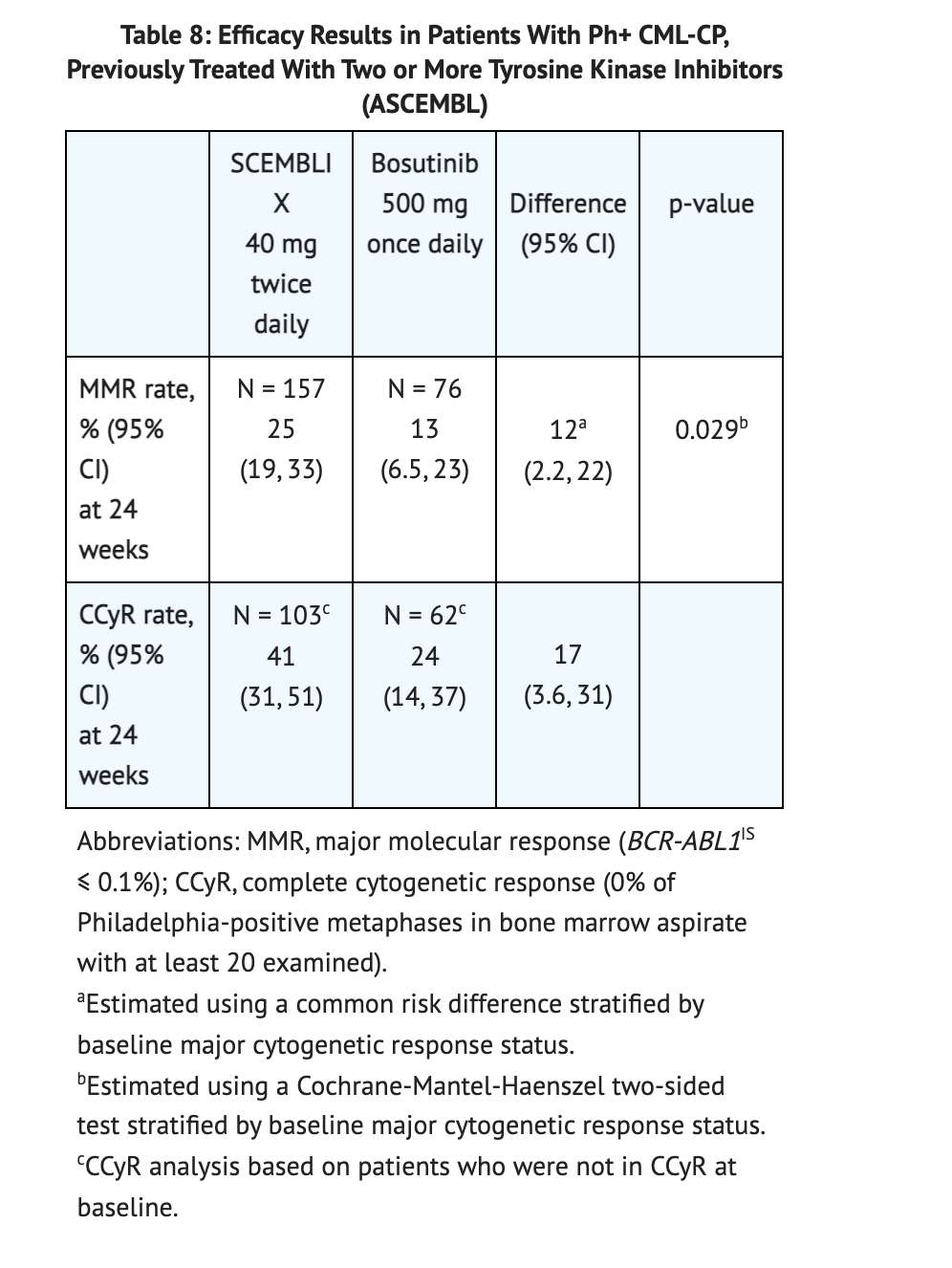
- The efficacy of Asciminib was tested on 233 patients that either received 40 mg of Asciminib twice daily or bosutinib 500 mg once daily. The patient population was largely Caucasian (75%), and included 52% women, and 19% were 65 years or older. Patients in the study took Asciminib for an average duration of 67 weeks and Bosutinib for an average duration 30 weeks. The results of the study are summarized in table 8. The study showed that MMR rate was at 29% for patients taking Asciminib and 13% for patients taking Bosutinib.
Table 8 shows the clinical data conducted in Patients with Ph+ CML-CP, previously treated with two or more TKIs taking Asciminib compared to Bosutinib.
Patients with Ph+ CML-CP with the T315I mutation
- The efficacy of Asciminib was tested on 45 patients that received 200 mg of Asciminib twice daily. The patient population was largely Caucasian (47%), and included 80% male, and 31% were 65 years or older. Patients in the study took Asciminib for an average duration of 108 weeks. The study showed MMR rate at 24 weeks (42%) and 96 weeks (49%) of the patients taking Asciminib.
How Supplied
- 20 mg Asciminib coated tablets (60 tablets per bottle).
- 40 mg Asciminib coated tablets (60 tablets per bottle).
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
- To protect from moisture, store in original container.
Images
Drug Images
{{#ask: Page Name::Asciminib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

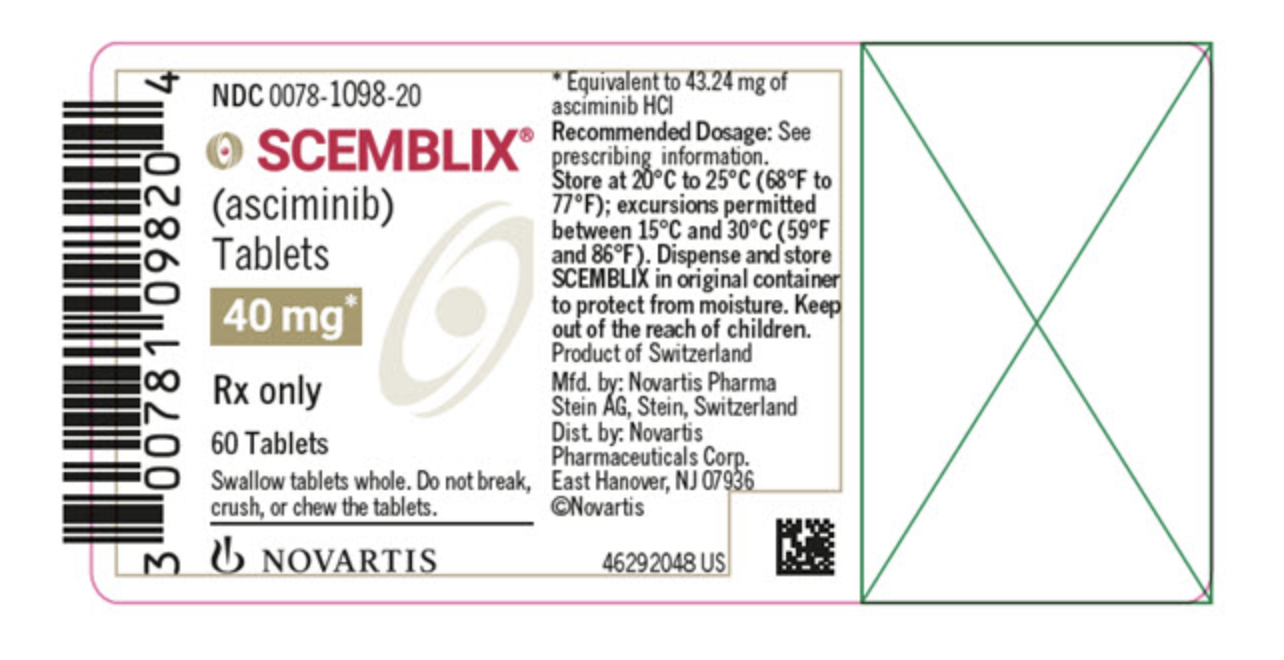
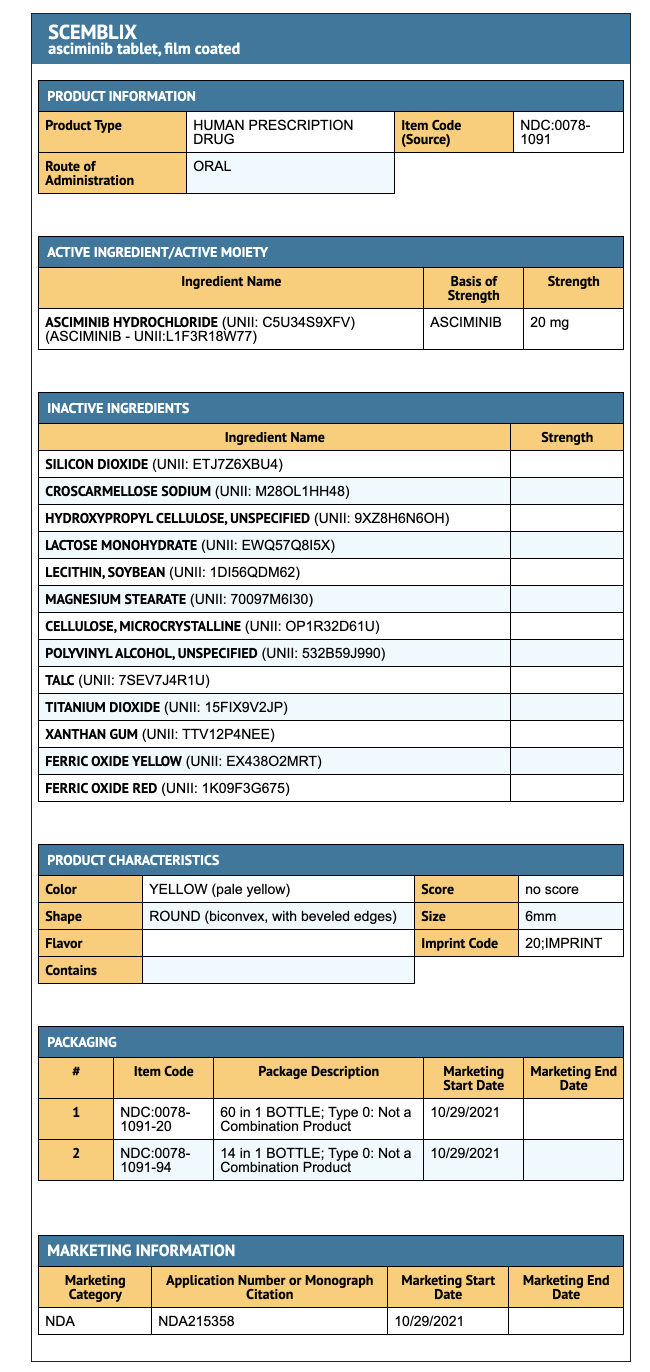
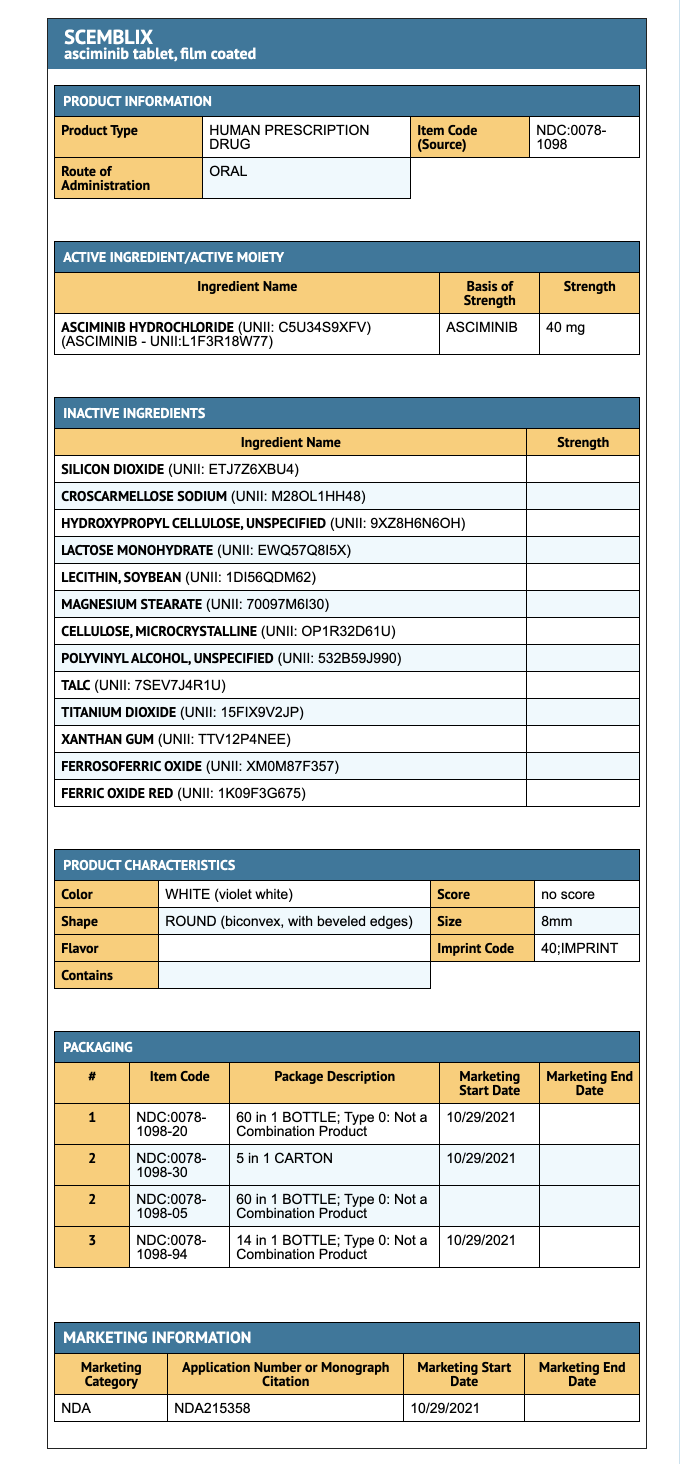
{{#ask: Label Page::Asciminib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Myelosuppression
- Asciminib may cause low blood cell counts.
- Patients should be advised to report any signs of bleeding and fever caused by this drug.
Pancreatic Toxicity
- Patients should be advised that pancreatitis may develop accompanied by symptoms such as nausea, vomiting, severe abdominal pain, or abdominal discomfort. Immediately seek medical attention if these symptoms occur.
Hypertension
- Patients should be advised that hypertension may develop along with symptoms such as increased blood pressure, confusion, headache, dizziness, chest pain, or shortness of breath which should be reported to healthcare provider when they persist.
Hypersensitivity
- If symptoms of hypersensitivity including rash, edema, or bronchospasm persist, immediately seek medical attention and stop use of Asciminib.
Cardiovascular Toxicity
- Inform patients that display symptoms of cardiovascular toxicity to seek medical help immediately.
Embryo Fetal Toxicity
- Female patients should be advised about the potential risks that may occur to fetus when taking Aciminib.
- Advise females of reproductive potential to use effective contraception during treatment with Asciminib and for at least 1 week after the last dose.
Lactation
- Inform female patients not to breastfeed when taking Asciminib and 1 week after last dose.
Drug Interactions
- The use of Asciminib and other medications can possibly alter side effects of Asciminib.
Instruction for taking Asciminib
- Take Asciminib orally either once daily or twice daily depending on the dosage prescribed.
- Inform patients to not alter dosage and schedule of each dosage unless told by healthcare provider.
- Swallow tablets whole and do not break or chew tablet.
- Patients advised not to eat two hours before taking tablet and one hour after taking tablet.
- For pateints that take Asciminib once daily, if a dosage is missed by more than 12 hours, then skip dosage and take next dosage at scheduled time.
- For pateints that take Asciminib twice daily, if a dosage is missed by more than 6 hours, then skip dosage and take next dosage at scheduled time.
Precautions with Alcohol
Alcohol-Asciminib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Scemblix
Look-Alike Drug Names
There is limited information regarding Asciminib Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.