Amoxicillin clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Clinical Pharmacology
Amoxicillin is stable in the presence of gastric acid and is rapidly absorbed after oral administration. The effect of food on the absorption of amoxicillin from amoxicillin tablets and amoxicillin suspension has been partially investigated. The 400 mg and 875 mg formulations have been studied only when administered at the start of a light meal. However, food effect studies have not been performed with the 200 mg and 500 mg formulations. Amoxicillin diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid, except when meninges are inflamed. The half-life of amoxicillin is 61.3 minutes. Most of the amoxicillin is excreted unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid. In blood serum, amoxicillin is approximately 20% protein-bound.
Orally administered doses of 250 mg and 500 mg amoxicillin capsules result in average peak blood levels 1 to 2 hours after administration in the range of 3.5 mcg/mL to 5 mcg/mL and 5.5 mcg/mL to 7.5 mcg/mL, respectively.
Mean amoxicillin pharmacokinetic parameters from an open, two-part, single-dose crossover bioequivalence study in 27 adults comparing 875 mg of amoxicillin with 875 mg of amoxicillin/ clavulanate potassium showed that the 875 g tablet of amoxicillin produces an AUC0-∞ of 35.4 ±8.1 mcg.hr/mL and a Cmax of 13.8 ±4.1 mcg/mL. Dosing was at the start of a light meal following an overnight fast.
Orally administered doses of amoxicillin suspension, 125 mg/5 mL and 250 mg/5 mL, result in average peak blood levels 1 to 2 hours after administration in the range of 1.5 mcg/mL to 3 mcg/mL and 3.5 mcg/mL to 5 mcg/mL, respectively.
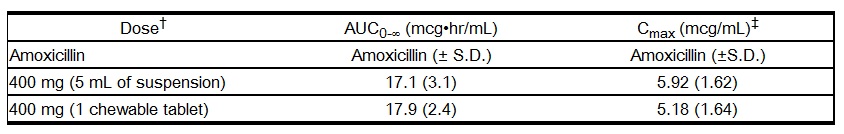
Oral administration of single doses of 400 mg amoxicillin chewable tablets and 400 mg/5 mL suspension to 24 adult volunteers yielded comparable pharmacokinetic data:
|
† Administered at the start of a light meal.
‡ Mean values of 24 normal volunteers. Peak concentrations occurred approximately 1 hour after the dose.
Detectable serum levels are observed up to 8 hours after an orally administered dose of amoxicillin. Following a 1 gram dose and utilizing a special skin window technique to determine levels of the antibiotic, it was noted that therapeutic levels were found in the interstitial fluid. Approximately 60% of an orally administered dose of amoxicillin is excreted in the urine within 6 to 8 hours.[1]
References
Adapted from the FDA Package Insert.