Amoxicillin-clavulanate potassium dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Dosage and Administration
AUGMENTIN may be taken without regard to meals; however, absorption of clavulanate potassium is enhanced when AUGMENTIN is administered at the start of a meal. To minimize the potential for gastrointestinal intolerance, AUGMENTIN should be taken at the start of a meal.
Adults
The usual adult dose is one 500-mg tablet of AUGMENTIN every 12 hours or one 250-mg tablet of AUGMENTIN every 8 hours. For more severe infections and infections of the respiratory tract, the dose should be one 875-mg tablet of AUGMENTIN every 12 hours or one 500-mg tablet of AUGMENTIN every 8 hours. Adults who have difficulty swallowing may be given the 125 mg/5 mL or 250 mg/5 mL suspension in place of the 500-mg tablet. The 200 mg/5 mL suspension or the 400 mg/5 mL suspension may be used in place of the 875-mg tablet.
Two 250-mg tablets of AUGMENTIN should not be substituted for one 500-mg tablet of AUGMENTIN. Since both the 250-mg and 500-mg tablets of AUGMENTIN contain the same amount of clavulanic acid (125 mg, as the potassium salt), two 250-mg tablets are not equivalent to one 500-mg tablet of AUGMENTIN.
The 250-mg tablet of AUGMENTIN and the 250-mg chewable tablet should not be substituted for each other, as they are not interchangeable. The 250-mg tablet of AUGMENTIN and the 250-mg chewable tablet do not contain the same amount of clavulanic acid (as the potassium salt). The 250-mg tablet of AUGMENTIN contains 125 mg of clavulanic acid, whereas the 250-mg chewable tablet contains 62.5 mg of clavulanic acid.
Pediatric Patients
Based on the amoxicillin component, AUGMENTIN should be dosed as follows:
- Neonates and Infants Aged <12 weeks (<3 months): The recommended dose of AUGMENTIN is 30 mg/kg/day divided every 12 hours, based on the amoxicillin component. Experience with the 200 mg/5 mL formulation in this age group is limited, and thus, use of the 125 mg/5 mL oral suspension is recommended.
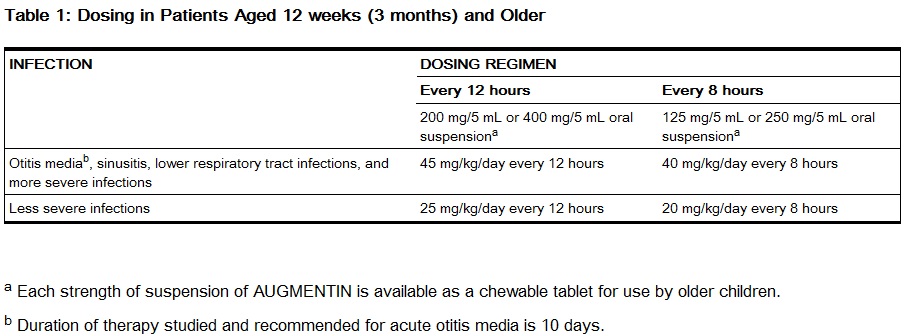
- Patients Aged 12 weeks (3 months) and Older: See dosing regimens provided in Table 1. The every 12 hour regimen is recommended as it is associated with significantly less diarrhea [see Clinical Studies (14.2)]. However, the every 12 hour suspension (200 mg/5 mL and 400 mg/5 mL) and chewable tablets (200 mg and 400 mg) contain aspartame and should not be used by phenylketonurics. [see Warnings and Precautions]
Patients Weighing 40 kg or More: Pediatric patients weighing 40 kg or more should be dosed according to adult recommendations.
The 250-mg tablet of AUGMENTIN should not be used until the child weighs at least 40 kg,due to the different amoxicillin to clavulanic acid ratios in the 250-mg tablet of AUGMENTIN (250/125) versus the 250-mg chewable tablet of AUGMENTIN (250/62.5).
Patients with Renal Impairment
Patients with impaired renal function do not generally require a reduction in dose unless the impairment is severe. Renal impairment patients with a glomerular filtration rate of <30 mL/min should not receive the 875‑mg dose. Patients with a glomerular filtration rate of 10 to 30 mL/min should receive 500 mg or 250 mg every 12 hours, depending on the severity of the infection. Patients with a glomerular filtration rate less than 10 mL/min should receive 500 mg or 250 mg every 24 hours, depending on severity of the infection.
Hemodialysis patients should receive 500 mg or 250 mg every 24 hours,depending on severity of the infection. They should receive an additional dose both during and at the end of dialysis.
Directions for Mixing Oral Suspension
Prepare a suspension at time of dispensing as follows: Tap bottle until all the powder flows freely. Add approximately 2/3 of the total amount of water for reconstitution (see Table 2 below) and shake vigorously to suspend powder. Add remainder of the water and again shake vigorously.
- Note: Shake oral suspension well before using. Reconstituted suspension must be stored under refrigeration and discarded after 10 days.[1]
References
Adapted from the FDA Package Insert.