Amantadine dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Dosage And Administration
The dosage of Amantadine Hydrochloride Capsules may need to be reduced in patients with congestive heart failure, peripheral edema, orthostatic hypotension, or impaired renal function (see Dosage for Impaired Renal Function).
Dosage for Prophylaxis and Treatment of Uncomplicated Influenza A Virus Illness
Adult: The adult daily dosage of Amantadine Hydrochloride Capsules is 200 mg; two 100 mg capsules as a single daily dose. The daily dosage may be split into one capsule of 100 mg twice a day. If central nervous system effects develop in once-a-day dosage, a split dosage schedule may reduce such complaints. In persons 65 years of age or older, the daily dosage of Amantadine Hydrochloride Capsules is 100 mg.
A 100 mg daily dose has also been shown in experimental challenge studies to be effective as prophylaxis in healthy adults who are not at high risk for influenza-related complications. However, it has not been demonstrated that a 100 mg daily dose is as effective as a 200 mg daily dose for prophylaxis, nor has the 100 mg daily dose been studied in the treatment of acute influenza illness. In recent clinical trials, the incidence of central nervous system (CNS) side effects associated with the 100 mg daily dose was at or near the level of placebo. The 100 mg dose is recommended for persons who have demonstrated intolerance to 200 mg of Amantadine Hydrochloride Capsules daily because of CNS or other toxicities.
Pediatric Patients: 1 yr. to 9 yrs. of age: The total daily dose should be calculated on the basis of 2 to 4 mg/lb/day (4.4 to 8.8 mg/kg/day), but not to exceed 150 mg per day.
9 yrs.to12 yrs. of age: The total daily dose is 200 mg given as one capsule of 100 mg twice a day. The 100 mg daily dose has not been studied in this pediatric population. Therefore, there are no data which demonstrate that this dose is as effective as or is safer than the 200 mg daily dose in this patient population.
Prophylactic dosing should be started in anticipation of an influenza A outbreak and before or after contact with individuals with influenza A virus respiratory tract illness.
Amantadine Hydrochloride Capsules should be continued daily for at least 10 days following a known exposure. If Amantadine Hydrochloride Capsules are used chemoprophylactically in conjunction with inactivated influenza A virus vaccine until protective antibody responses develop, then it should be administered for 2 to 4 weeks after the vaccine has been given. When inactivated influenza A virus vaccine is unavailable or contraindicated, Amantadine Hydrochloride Capsules should be administered for the duration of known influenza A in the community because of repeated and unknown exposure.
Treatment of influenza A virus illness should be started as soon as possible, preferably within 24 to 48 hours after onset of signs and symptoms, and should be continued for 24 to 48 hours after the disappearance of signs and symptoms.
Dosage for Parkinsonism
Adult: The usual dose of Amantadine Hydrochloride Capsules is 100 mg twice a day when used alone. Amantadine Hydrochloride Capsules have an onset of action usually within 48 hours.
The initial dose of Amantadine Hydrochloride Capsules is 100 mg daily for patients with serious associated medical illnesses or who are receiving high doses of other antiparkinson drugs. After one to several weeks at 100 mg once daily, the dose may be increased to 100 mg twice daily, if necessary.
Occasionally, patients whose responses are not optimal with Amantadine Hydrochloride Capsules at 200 mg daily may benefit from an increase up to 400 mg daily in divided doses. However, such patients should be supervised closely by their physicians.
Patients initially deriving benefit from Amantadine Hydrochloride Capsules not uncommonly experience a fall-off of effectiveness after a few months. Benefit may be regained by increasing the dose to 300 mg daily. Alternatively, temporary discontinuation of Amantadine Hydrochloride Capsules for several weeks, followed by reinitiation of the drug, may result in regaining benefit in some patients. A decision to use other antiparkinson drugs may be necessary.
Dosage for Concomitant Therapy
Some patients who do not respond to anticholinergic antiparkinson drugs may respond to Amantadine Hydrochloride Capsules. When Amantadine Hydrochloride Capsules or anticholinergic antiparkinson drugs are each used with marginal benefit, concomitant use may produce additional benefit.
When Amantadine Hydrochloride Capsules and levodopa are initiated concurrently, the patient can exhibit rapid therapeutic benefits. Amantadine Hydrochloride Capsules, should be held constant at 100 mg daily or twice daily while the daily dose of levodopa is gradually increased to optimal benefit.
When Amantadine Hydrochloride Capsules are added to optimal well-tolerated doses of levodopa, additional benefit may result, including smoothing out the fluctuations in improvement which sometimes occur in patients on levodopa alone. Patients who require a reduction in their usual dose of levodopa because of development of side effects may possibly regain lost benefit with the addition of Amantadine Hydrochloride Capsules.
Dosage for Drug-Induced Extrapyramidal Reactions
Adult: The usual dose of Amantadine Hydrochloride Capsules is 100 mg twice a day. Occasionally, patients whose responses are not optimal with Amantadine Hydrochloride Capsules at 200 mg daily may benefit from an increase up to 300 mg daily in divided doses.
Dosage for Impaired Renal Function
Depending upon creatinine clearance, the following dosage adjustments are recommended:
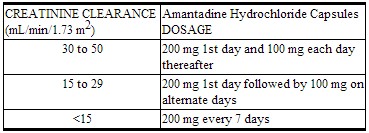 |
The recommended dosage for patients on hemodialysis is 200 mg every 7 days.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016023s041,018101s016lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.