Alpha particle radiation astronomy
Editor-In-Chief: Henry A. Hoff
Regarding alpha particles, as with beta and gamma particles/rays, the name used for the particle carries some mild connotations about its production process and energy, but these are not rigorously applied.[1]
Radiation
Alpha radiation is an average of about 20 times more dangerous, and in experiments with inhaled alpha emitters, up to 1000 times more dangerous[2] than an equivalent activity of beta emitting or gamma emitting radioisotopes.
A given dose of alpha-particles inhaled presents the same risk as a 20-times higher dose of gamma radiation.[3] The powerful alpha emitter polonium-210 (a milligram of 210Po emits as many alpha particles per second as 4.215 grams of 226Ra) is suspected of playing a role in lung cancer and bladder cancer related to tobacco smoking.[4] 210Po was used to kill Russian dissident and ex-Federal Security Service of the Russian Federation (FSB) officer Alexander V. Litvinenko in 2006.[5]
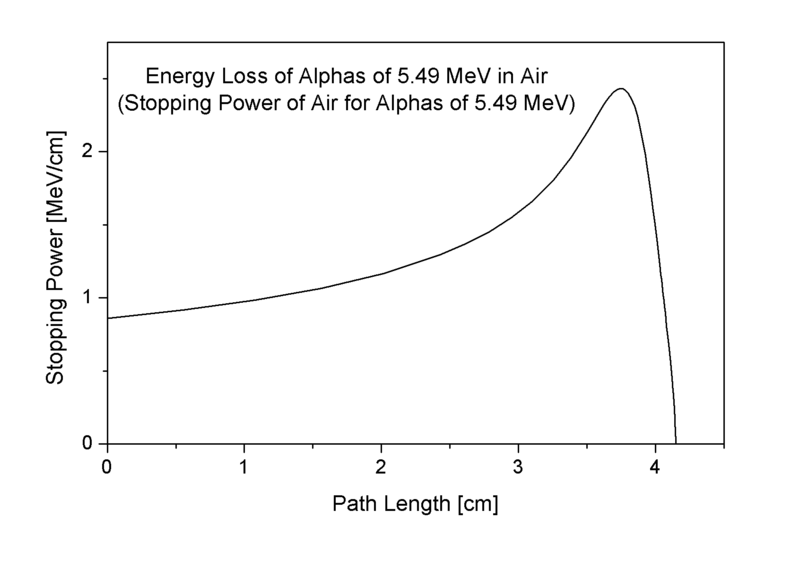
According to the energy-loss curve, first image on the right, it is recognizable that the alpha particle indeed loses more energy on the end of the trace.[6]
Theoretical alpha particles
Def. a "positively charged Helium nucleus [nucleus of a helium-4 atom][7] (consisting of two protons and two neutrons), emitted as a consequence of radioactivity"[8] is called an alpha particle, or α-particle.
Sources
Sources of noise, such as alpha and beta particles, can be eliminated by various shielding materials, such as lead, plastic, thermo-coal, etc. Thus, photons cause major interference in neutron detection, since it is uncertain if neutrons or photons are being detected by the neutron detector.
While in the past radium and radon have both been used for radiography, they have fallen out of use as they are radiotoxic alpha radiation emitters which are expensive; iridium-192 and cobalt-60 are far better photon sources.
Regarding the straggling of alpha particles in metal foils, "the Bi'" and Am'" sources each have two closely spaced alpha-particle groups and the Pu'" source has three such groups, it was found that the resolution obtained with the apparatus enabled one to obtain the location of the dominant group in each source with great accuracy."[9]
Emissions
Alpha decay is characterized by the emission of an alpha particle, a 4He nucleus. The mode of this decay causes the parent nucleus to decrease by two protons and two neutrons. This type of decay follows the relation:
<math>{}_Z^A\!X\to {}_{Z-2}^{A-4}\!Y+ {}_4^2\alpha</math> [10]
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha particles. The neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.
Proton emission (also known as proton radioactivity) is a type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying [nuclear isomer] isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay.
Beta decay is characterized by the emission of a neutrino and a negatron which is equivalent to an electron. This process occurs when a nucleus has an excess of neutrons with respect to protons, as compared to the stable isobar. This type of transition converts a neutron into a proton; similarly, a positron is released when a proton is converted into a neutron. These decays follows the relation:
<math>{}_Z^A\!X\to {}_{Z+1}^A\!Y+ \bar{\nu} + \beta^-</math>
<math>{}_Z^A\!X\to {}_{Z-1}^A\!Y+ \nu + \beta^+</math> [11]
Gamma ray emission is follows the previously discussed modes of decay when the decay leaves a daughter nucleus in an excited state. This nucleus is capable of further de-excitation to a lower energy state by the release of a photon. This decay follows the relation:
<math>{}^A\!X^* \to {}^A\!Y + \gamma </math>[12]
Generation of electromagnetic radiation can occur whenever charged particles pass within certain distances of each other without being in fixed orbits, the accelerations (or decelerations) may give off the radiation. This is partly illustrated by the diagram at right where an electron has its course altered by near passage by a positive particle. Bremsstrahlung radiation also occurs when two electrons or other similarly charged particles pass close enough to deflect, slow down, or speed up at least one of the particles.
Bremsstrahlung includes synchrotron and cyclotron radiation.
When high-energy radiation bombards materials, the excited atoms within emit characteristic "secondary" (or fluorescent) radiation.
Reflections
{{free media}}Alpha particles can be scattered by more than 90°.[13]
In the diagram on the right, alpha particles emitted by a radioactive source (A) were observed bouncing off a metal reflector (R) and onto a fluorescent screen (S) on the other side of a lead plate.[13]
The alpha particle emitter consists of a small conical glass tube containing "radium emanation" (radon), "radium A" (actual radium), and "radium C" (bismuth-214); its open end sealed with mica.[13]
The tube was held on the opposite side of plate, such that the alpha particles it emitted could not directly strike the screen.[13]
By pointing the tube at the foil the alpha particles would bounce off it and strike the screen (S) on the other side of the plate, and observed an increase in the number of scintillations on the screen. Counting the scintillations, metals with higher atomic mass, such as gold, reflected more alpha particles than lighter ones such as aluminium.[13]
Scatterings
In the image on the right is an apparatus used to observe the scattering of alpha particles by a metal foil: the long glass tube, nearly two meters in length, at one end was a quantity of "radium emanation" (R) that served as a source of alpha particles, the opposite end of the tube was covered with a phosphorescent screen (Z); in the middle of the tube was a 0.9 mm-wide slit.[14]
The alpha particles from R passed through the slit and created a glowing patch of light on the screen; a microscope (M) was used to count the scintillations on the screen and measure their spread.[14]
By pumping all the air out of the tube the alpha particles would be unobstructed, and they left a neat and tight image on the screen that corresponded to the shape of the slit.[14] By allowing some air in the tube, the glowing patch became more diffuse; then by pumping out the air and placing some gold foil over the slit at AA, this too caused the patch of light on the screen to become more spread out demonstrating that both air and solid matter could markedly scatter alpha particles.[14]
The central charge of the atom was assumed positive, but a negative charge would have fitted the scattering model just as well.[15]
The passage of alpha particles through gases such as hydrogen and nitrogen: a beam of alpha particles was shot through hydrogen, the alpha particles knocked the hydrogen nuclei forwards in the direction of the beam, not backwards.[15]
In another experiment alpha particles were shot through nitrogen, the alpha particles knocked hydrogen nuclei (i.e. protons) out of the nitrogen nuclei.[15]
Diffractions
To measure how the most probable angle through which an a-particle is deflected varies with the material it passes through, the thickness of said material, and the velocity of the alpha particles, construct an airtight glass tube from which the air was pumped out; at one end place a bulb (B) containing "radium emanation" (radon-222); by means of mercury, the radon in B was pumped up the narrow glass pipe whose end at A was plugged with mica; at the other end of the tube was a fluorescent zinc sulfide screen (S); affix the microscope used to count the scintillations on the screen to a vertical millimeter scale with a vernier, which allows precise measurement where the flashes of light appear on the screen to calculate the particles' angles of deflection.[16]
The alpha particles emitted from A was narrowed to a beam by a small circular hole at D, by placing a metal foil in the path of the rays at D and E observe how the zone of flashes changes.[16] Vary the velocity of the alpha particles by placing extra sheets of mica or aluminium at A.[16]
Nuclear scatterings
The atom contains at its center a volume of electric charge that is very small and intense.[17]
How a foil should scatter the alpha particles if all the positive charge and most of the atomic mass was concentrated in a single point at the center of an atom is given by:[17]
<math> s = \frac {Xnt\csc^4{\!\left(\tfrac {\phi}{2}\right)}}{16r^2} \cdot {\left(\frac {2Q_n Q_{\alpha}}{mv^2}\right)}^2 </math>
- s = the number of alpha particles falling on unit area at an angle of deflection Φ
- r = distance from point of incidence of α rays on scattering material
- X = total number of particles falling on the scattering material
- n = number of atoms in a unit volume of the material
- t = thickness of the foil
- Qn = positive charge of the atomic nucleus
- Qα = positive charge of the alpha particles
- m = mass of an alpha particle
- v = velocity of the alpha particle
From the scattering data, the central charge Qn was estimated to be about +100 units.[17]
Nuclear diffractions
Rutherford's equation predicted that the number of scintillations per minute s that will be observed at a given angle Φ should be proportional to:[17]
- csc4(Φ/2)
- thickness of foil t
- magnitude of central charge Qn
- 1/(mv2)2
To test how the scattering varied with the angle of deflection (i.e. if s ∝ csc4(Φ/2)) an apparatus that consists of a hollow metal cylinder mounted on a turntable was built, where inside the cylinder was a metal foil (F) and a radiation source containing radon (R), mounted on a detached column (T) which allowed the cylinder to rotate independently.[18]
The column was also a tube by which air was pumped out of the cylinder, with a microscope (M), its objective lens covered by a fluorescent zinc sulfide screen (S) penetrated the wall of the cylinder and pointed at the metal foil.[18]
By turning the table, the microscope could be moved a full circle around the foil, allowing observation and counting alpha particles deflected by up to 150°; correcting for experimental error, the number of alpha particles that are deflected by a given angle Φ is indeed proportional to csc4(Φ/2).[18]
In the second set of images on the right, how the scattering varied with the thickness of the foil (i.e. if s ∝ t) was tested by constructing a disc (S) with six holes drilled in it, where the holes were covered with metal foil (F) of varying thickness, or none for control.[18]
The disc was then sealed in a brass ring (A) between two glass plates (B and C), could be rotated by means of a rod (P) to bring each window in front of the alpha particle source (R), where on the rear glass pane was a zinc sulfide screen (Z).[18]
The number of scintillations that appeared on the zinc sulfide screen was proportional to the thickness as long as this thickness was small.[18]
To measure how the scattering pattern varied with the square of the nuclear charge (i.e. if s ∝ Qn2), assume it was proportional to the atomic weight, so it was tested whether the scattering was proportional to the atomic weight squared.[18]
Cover the holes of the disc with foils of gold, tin, silver, copper, and aluminum; measure each foil's stopping power by equating it to an equivalent thickness of air; count the number of scintillations per minute that each foil produced on the screen; divide the number of scintillations per minute by the respective foil's air equivalent, then divide again by the square root of the atomic weight (for foils of equal stopping power, the number of atoms per unit area is proportional to the square root of the atomic weight).[18]
Thus, for each metal, the number of scintillations that a fixed number of atoms produce was obtained; then for each metal, divide this number by the square of the atomic weight, and to show that the ratios were more or less the same proving that s ∝ Qn2.[18]
To test how the scattering varied with the velocity of the alpha particles (i.e. if s ∝ 1/v4), use the same apparatus again, and slow the alpha particles by placing extra sheets of mica in front of the alpha particle source.[18]
Within the range of experimental error, that the number of scinitillations was proportional to 1/v4.[18]
Cosmic rays
About 89% of cosmic rays are simple protons or hydrogen nuclei, 10% are helium nuclei or alpha particles, and 1% are the nuclei of heavier elements. Solitary electrons constitute much of the remaining 1%.
"The energy spectra of solar protons and helium nuclei [the two most abundant components in solar cosmic rays] have been measured simultaneously on several occasions using nuclear emulsion detectors flown on balloons [...]."[19]
These "have different velocity spectra, similar, but not exactly identical rigidity spectra, and varying relative abundances."[19]
"The multiply charged nuclei, on the other hand, appear to have the same spectral shape and relative abundances each time measurements are made, at least in the region from 42 to 135 MeV/nucleon. Further, these relative abundances seem to reflect those of the solar atmosphere insofar as comparison can be made."[19]
"Finally, there is positive evidence that very small quantities of deuterons exist, probably in an amount which is about 10-3 or less of the proton abundance."[19]
The "rare components [are] deuterons, tritons, He3 nuclei, electrons, neutrons, and the heavier nuclei."[19]
Alpha decays
A table of nuclides or chart of nuclides is a two-dimensional [Cartesian coordinate system] graph in which one axis represents the number of neutrons and the other represents the number of protons in an atomic nucleus. Each point plotted on the graph thus represents the nuclide of a real or hypothetical chemical element. Hydrogen is at the lower left.
"Natural alpha decay takes place in heavy nuclei [...]. Each alpha decay leads to ΔΛ = 4, ΔZ = 2. Since this tends to move nuclei off the line of beta stability to the neutron-rich side, beta (-minus) decays are found in conjunction with alpha decays. There are thus four series (or chains) of alpha decays into which the natural alpha decays can be fitted; these correspond to Λ = 4n, 4n + 1, 4n + 2 and 4n + 3, where n is an integer.
- 4n
- Thorium series: 232
Th ➙ 208
Pb - 4n+1
- Neptunium series: 237
Np ➙ 209
Bi - 4n+2
- Uranium series: 238
U ➙ 206
Pb - 4n+3
- Actinium series: 235
U ➙ 207
Pb"[20]
"A decay starting with the heaviest nucleus in a series can continue down to the lightest, with a sequence of alpha and beta decays following roughly the line of stability."[20]
"It follows from conservation of energy and momentum that the kinetic energy of the alpha and the residual nucleus has a fixed value. Alpha particles are thus emitted with a sharply peaked spectrum."[20]
Alpha decay results from the Coulomb repulsion[21] between the alpha particle and the rest of the nucleus, which both have a positive electric charge, but which is kept in check by the nuclear force.
Alpha nuclides
Alpha nuclides have equal, even numbers of protons and neutrons; they are important in stellar nucleosynthesis since the energetic environment within stars is amenable to fusion of alpha particles into heavier nuclei.[22][23]
Alpha nuclide is also shorthand for alpha radionuclide, referring to those radioactive isotopes that undergo alpha decay and thereby emit alpha particles.[24]
List of alpha nuclides
| Alpha number | nuclide | Stable/radioactive | decay mode | half-life[25] | product(s) of decay (bold is stable) | notes |
|---|---|---|---|---|---|---|
| 1 | Template:Nuclide | Stable | ||||
| 2 | Template:Nuclide | Radioactive | α | 6.7(17)×10−17 s | Template:Nuclide | |
| 3 | Template:Nuclide | Stable | ||||
| 4 | Template:Nuclide | Stable | ||||
| 5 | Template:Nuclide | Stable | ||||
| 6 | Template:Nuclide | Stable | ||||
| 7 | Template:Nuclide | Stable | ||||
| 8 | Template:Nuclide | Stable | ||||
| 9 | Template:Nuclide | Observationally Stable | ||||
| 10 | Template:Nuclide | Observationally Stable | ||||
| 11 | Template:Nuclide | Radioactive | electron capture (EC) | 60.0(11) y | Template:Nuclide → Template:Nuclide | |
| 12 | Template:Nuclide | Radioactive | Beta decay (β+) | 21.56(3) h | Template:Nuclide → Template:Nuclide | |
| 13 | Template:Nuclide | Radioactive | β+ | 8.275(8) h | Template:Nuclide → Template:Nuclide | |
| 14 | Template:Nuclide | Radioactive | β+ | 6.075(10) d | Template:Nuclide → Template:Nuclide | |
| 15 | Template:Nuclide | Radioactive | Beta decay (β+) | 2.38(5) min | Template:Nuclide → Template:Nuclide |
Anti-alpha particles
In 2011, using the Relativistic Heavy Ion Collider at the U.S. Department of Energy's Brookhaven National Laboratory, the antimatter partner of the helium nucleus, also known as the anti-alpha, was detected.[26] The experiment used gold ions moving at nearly the speed of light and colliding head on to produce the antiparticle.[27]
Neutrons
Neutrons are produced when alpha particles impinge upon any of several low atomic weight isotopes including isotopes of lithium, beryllium, carbon and oxygen.
Heliums
The alpha process, also known as the alpha ladder is one of two classes of [nuclear] reactions [for converting] helium into heavier elements, the other being the triple-alpha process.[28]
Possible reactions:
- <math>\mathrm{_6^{12}C} + \mathrm{_2^4He} \rightarrow \mathrm{_{8}^{16}O} + \gamma + Q</math>, Q = 7.16 MeV
- <math>\mathrm{_8^{16}O} + \mathrm{_2^4He} \rightarrow \mathrm{_{10}^{20}Ne} + \gamma + Q</math>, Q = 4.73 MeV
- <math>\mathrm{_{10}^{20}Ne} + \mathrm{_2^4He} \rightarrow \mathrm{_{12}^{24}Mg} + \gamma + Q</math>, Q = 9.31 MeV
- <math>\mathrm{_{12}^{24}Mg} + \mathrm{_2^4He} \rightarrow \mathrm{_{14}^{28}Si} + \gamma + Q</math>, Q = 9.98 MeV
- <math>\mathrm{_{14}^{28}Si} + \mathrm{_2^4He} \rightarrow \mathrm{_{16}^{32}S} + \gamma + Q</math>, Q = 6.95 MeV
- <math>\mathrm{_{16}^{32}S} + \mathrm{_2^4He} \rightarrow \mathrm{_{18}^{36}Ar} + \gamma</math>
- <math>\mathrm{_{18}^{36}Ar} + \mathrm{_2^4He} \rightarrow \mathrm{_{20}^{40}Ca} + \gamma</math>
- <math>\mathrm{_{20}^{40}Ca} + \mathrm{_2^4He} \rightarrow \mathrm{_{22}^{44}Ti} + \gamma</math>
- <math>\mathrm{_{22}^{44}Ti} + \mathrm{_2^4He} \rightarrow \mathrm{_{24}^{48}Cr} + \gamma</math>
- <math>\mathrm{_{24}^{48}Cr} + \mathrm{_2^4He} \rightarrow \mathrm{_{26}^{52}Fe} + \gamma</math>
- <math>\mathrm{_{26}^{52}Fe} + \mathrm{_2^4He} \rightarrow \mathrm{_{28}^{56}Ni} + \gamma</math>
- <math>\mathrm{_{28}^{56}Ni} + \mathrm{_2^4He} + \gamma \rightarrow \mathrm{_{30}^{60}Zn}</math>
Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of the alpha particle). Alpha elements are atomic number Z ≤ 22: (C, N), O, Ne, Mg, Si, S, Ar, Ca, Ti. They may be synthesized by alpha-capture. Silicon and calcium are purely alpha process elements. Magnesium can be burned by proton capture reactions. Oxygen enhancement is well correlated with an enhancement of other alpha process elements. C and N are considered alpha process elements, when they are synthesized in nuclear alpha-capture reactions.
The abundance of alpha elements in stars is usually expressed in a logarithmic manner:
- <math> [\alpha/Fe] = \log_{10}{\left(\frac{N_{\alpha}}{N_{Fe}}\right)_{Star}} - \log_{10}{\left(\frac{N_{\alpha}}{N_{Fe}}\right)_{Sun}} </math>,
Here <math>N_{\alpha}</math> and <math>N_{Fe}</math> are the number of alpha element atoms and Fe atoms per unit volume.
- <math>\mathrm{_1^1H} + \mathrm{_1^1H} \rightarrow \mathrm{_{2}^{2}He}</math>
Berylliums
A photon carrying 1.67 MeV or more energy can photodisintegrate an atom of beryllium-9 (100% of natural beryllium, its only stable isotope):
- Template:Nuclide (γ,n) 2α
Antimony-124 is assembled with beryllium to make laboratory neutron sources and startup neutron sources. Antimony-124 (half-life 60.20 days) emits β− and 1.690 MeV gamma rays (also 0.602 MeV and 9 fainter emissions from 0.645 to 2.090 MeV), yielding stable tellurium-124. Gamma rays from Antimony-124 knock neutrons off beryllium-9 with an average kinetic energy of 24keV, intermediate neutrons. The other product is two alpha particles.[29][30]
Other isotopes have higher thresholds for photoneutron production, as high as 18.72 MeV, for carbon-12.[31]
Carbons
The triple alpha process is a set of [nuclear] reactions by which three ... [alpha particles] are transformed into carbon.[22][23]
Some of these reactions are
- <math>\mathrm{_2^4He} + \mathrm{_2^4He} \rightarrow \mathrm{_{4}^{8}Be}</math> (−93.7 keV) and
- <math>\mathrm{_4^8Be} + \mathrm{_2^4He} \rightarrow \mathrm{_{6}^{12}C}</math> (+7.367 MeV).
The net energy release of the process is 1.166 pJ.
An additional alpha process reaction that may occur is
- <math>\mathrm{_{6}^{12}C} + \mathrm{_2^4He} \rightarrow \mathrm{_{8}^{16}O} + \gamma</math> (+7.162 MeV).
4He(αα,γ)12C or
- <math>\mathrm{_2^4He} + \mathrm{_2^4He} \rightarrow \mathrm{_{4}^{8}Be}</math> (−93.7 keV) and
- <math>\mathrm{_4^8Be} + \mathrm{_2^4He} \rightarrow \mathrm{_{6}^{12}C}</math> (+7.367 MeV).
14N(n,p)14C
Nitrogens
The carbon-burning process is a set of nuclear reactions that may require high temperatures (> 5×108 K or 50 keV) and densities (> 3×109 kg/m3).[32]
CNO-I
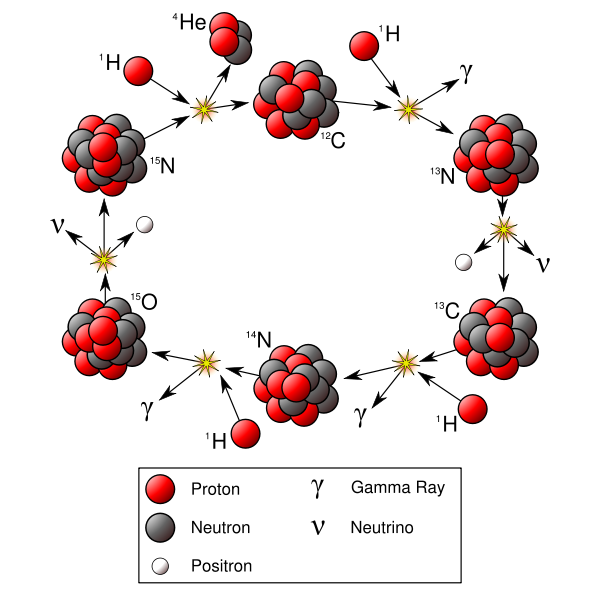
The principal reactions are:[33]"
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 1.95 MeV
Template:Nuclide2 → Template:Nuclide2 + e+ + Template:SubatomicParticle + 1.20 MeV (half-life of 9.965 minutes[34]), or 12C(p,γ)13N.
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 7.54 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 7.35 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 1.73&bsp;MeV (half-life of 122.24 seconds[34])
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 4.96 MeV:[21]
CNO-II
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 12.13 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 0.60 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.76 MeV (half-life of 64.49 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 1.19 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 7.35 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.75 MeV (half-life of 122.24 seconds)
CNO-III
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 5.61 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 1.656 MeV (half-life of 109.771 minutes)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 3.98 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 12.13 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 0.60 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.76 MeV (half-life of 64.49 seconds)
CNO-IV
These reactions may occur in massive stars. Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 8.114 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 0.60 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.76 MeV (half-life of 64.49 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 5.61 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 1.656 MeV (half-life of 109.771 minutes)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 7.994 MeV
These are the hot CNO cycles reactions with conditions of higher temperature as have been found in novae and X-ray bursts.
When the rate of proton captures exceeds the rate of beta-decay, the burning conforms to the proton drip line. A radioactive species captures a proton before it can beta decay.
HCNO-I
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 1.95 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 4.63 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 5.14 MeV (half-life of 70.641 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 7.35 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.75 MeV (half-life of 122.24 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 4.96 MeV
HCNO-II
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 12.13 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 0.60 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 3.92 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 4.44 MeV (half-life of 1.672 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 2.88 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 2.75 MeV (half-life of 122.24 seconds)
HCNO-III
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 6.41 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 3.32 MeV (half-life of 17.22 seconds)
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + Template:Nuclide2 + 8.11 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 0.60 MeV
Template:Nuclide2 + Template:Nuclide2 → Template:Nuclide2 + γ + 3.92 MeV
Template:Nuclide2 → Template:Nuclide2 + Template:SubatomicParticle + Template:SubatomicParticle + 4.44 MeV (half-life of 1.672 seconds)
Borophosphosilicate glasses
Borophosphosilicate glass, commonly known as BPSG, is a type of silicate glass that includes additives of both boron and phosphorus. Silicate glasses such as phosphosilicate glass (PSG) and borophosphosilicate glass are commonly used in semiconductor device fabrication for intermetal layers, i.e., insulating layers deposited between succeedingly higher metal or conducting layers.
BPSG has been implicated in increasing a device's susceptibility to soft errors since the boron-10 isotope is good at capturing thermal neutrons from cosmic radiation.[35][36] It then undergoes fission producing a gamma ray, an alpha particle, and a lithium ion. These products may then dump charge into nearby structures, causing data loss (bit flipping, or single event upset).
Bismuths
Because of the relatively low neutron fluxes expected to occur during the S-process (on the order of 105 to 1011 neutrons per cm2 per second), this process does not have the ability to produce any of the heavy radioactive isotopes such as thorium or uranium. The cycle that terminates the S-process is:
- 209Bi + n → 210Bi + γ
- <math>\mathrm{_{83}^{210}Bi} \to \mathrm{_{84}^{210}Po} + e^- + \bar\nu_e </math>
- Po → 206Pb + 4He
206Pb then captures three neutrons, producing 209Pb, which decays to 209Bi by β- decay, restarting the cycle:
- 206Pb + 3n → 209Pb
- <math> \mathrm{_{82}^{209}Pb} \to \mathrm{_{83}^{209}Bi} + e^- + \bar\nu_e </math>
The net result of this cycle therefore is that 4 neutrons are converted into one alpha particle, two electrons, two anti-electron neutrinos and gamma radiation:
- <math>4n \to \mathrm{_2^{4}He} + 2e^- + 2\bar\nu_e + \gamma</math>
The process thus terminates in bismuth, the heaviest "stable" element. (Bismuth is actually slightly radioactive, but with a half-life so long—a billion times the present age of the universe—that it is effectively stable over the lifetime of any existing star.)
The following are among the principal radioactive materials known to emit alpha particles:
- Bismuth: 209Bi, 211Bi, 212Bi, 213Bi
- Polonium: 210Po, 211Po, 212Po, 214Po, 215Po, 216Po, 218Po
- Astatine: 215At, 217At, 218At
- Radon: 218Rn, 219Rn, 220Rn, 222Rn, 226Rn
- Francium: 221Fr
- Radium: 223Ra, 224Ra, 226Ra
- Actinium: 225Ac, 227Ac
- Thorium: 227Th, 228Th, 229Th, 230Th, 232Th
- Protactinium: 231Pa
- Uranium: 233U, 234U, 235U, 236U, 238U
- Neptunium: 237Np
- Plutonium: 238Pu, 239Pu, 240Pu, 244Pu
- Americium: 241Am
- Curium: 244Cm, 245Cm, 248Cm
- Californium: 249Cf, 252Cf
Moon
{{free media}}"Radon-222 is a radioactive noble gas which is produced during the decay of uranium. Because it is a noble gas, under certain conditions it may diffuse through the lunar regolith and reach the surface before decaying by alpha particle emission. The alpha particle spectrometers aboard the Apollo 15 and 16 spacecrafts were designed to detect and identify the alpha particles emitted by 222Rn and her daughter products during decay."[37]
"The radon is produced at the site of its parents, i.e., within mineral grains. In order for the radon to read the surface two distinct processes must occur. First the radon bus be released to the voids between the mineral grains. This process is commonly referred to as emanation. Emanation can occur by diffusion within the solid grains or by recoil out of the grain from the alpha decay of its parent. Neither process is expected to be efficient on th lunar surface. In particular, to have the recoil be efficient one requires a stopping medium between the mineral grains. On earth the atmosphere provides some stopping power. The lack of a lunar atmosphere must significantly reduce the efficiency of this process. [...] Once a neutral radon atom is in the lunar regolith it must migrate to the surface to be observable by the spectrometer. This can occur by a random walk through the regolith or by transport within some other medium passing through the regolith."[37]
"The distribution of 222Rn and her daughter products have been observed from orbit during the Apollo 15 and Apollo 16 missions. Decays of 222Rn were observed locally over the crater Aristarchus and more generally over Oceanus Procellarum and Mare Imbrium. Decays of 210Po (a delayed daughter of 222Rn) were observed over most of the eastern hemisphere on both missions. All observations indicate a lack of radioactive equilibrium between 222Rn and 210Po implying a time dependent process for the production of radon at the lunar surface. In addition, the 210Po shows a remarkable correlation with the Mare edges."[37]
"Lunar Explorer 35, a 104 kg spin stabilized spacecraft, was placed in lunar orbit on 22 July 1967 with period = 11.5 hours, inclination = 169°, aposelene = 9388 ± 100 km, periselene = 2568 ± 100 km, and initial aposelene-moon-sun angle = 304°E. The experiment repertoire includes magnetometers, plasma probes, energetic particle and cosmic dust detectors."[38]
"A study of the time history, intensity and angular distribution of solar emitted protons and alpha particles has been conducted for the period 19 July to 31 December 1967. A noteworthy finding is that during this period interplanetary space is seldom free of a measurable intensity (j ≥ 0.1/cm2sec sterad) of protons (Ep ≥ 322 keV)."[38]
"The absolute intensity and angular distribution of alpha particles 2.0 ≤ E𝛂 ≤ 10.2 meV has been obtained by a solid state detector making it possible to estimate the emissivity of alpha particles from the radioactive gases thoron and radon of the tenuous lunar atmosphere. A provisional upper limit from the analysis of two months of data is that 𝛔 ≤ 10-2/cm2sec sterad."[38]
Mars
Meteorites have been found on Mars.[39]
"This [image at right] is a false-color, red-green-blue composite view generated from images taken through the Pancam's 750-nanometer, 530-nanometer and 430-nanometer filters. The exaggeraged color is used for enhancing the visibility of differences among the types of rock and soil materials."[40]
"Analysis of Block Island's composition using the rover's alpha particle X-ray spectrometer confirmed that it is rich in iron and nickel. The rock is about 60 centimeters (2 feet) across."[40]
Imaged at lower right is an igneous Martian shergottite meteorite. "The perimeter exhibits a fusion crust from the heat of entry into the Earth’s atmosphere. It is a fresh sample of NWA 6963, an igneous Martian shergottite meteorite found in September 2011 in Morocco. Meteorites are often labeled NWA for North West Africa, not because they land there more often, but because they are easy to spot as peculiar objects in the desert sands. From the geochemistry and presence of various isotopes, the origin and transit time is deduced. The 99 meteorites from Mars exhibit precise elemental and isotopic compositions similar to rocks and atmosphere gases analyzed by spacecraft on Mars, starting with the Viking lander in 1976. Compared to other meteorites, the Martians have younger formation ages, unique oxygen isotopic composition (consistent for Mars and not for Earth), and the presence of aqueous weathering products. A trapped gas analysis concluded that their origin was Mars quite recently, in the year 2000."[41]
"The formation ages of meteorites often come from their cosmic-ray exposure (CRE), measured from the nuclear products of interactions of the meteorite in space with energetic cosmic ray particles. This one is particularly young, having crystallized only 180 million years ago, suggesting that volcanic activity was still present on Mars at that time. Volcanic flows are the youngest part of a planet, and this one happened to be hit by a meteor impact, ejecting" it from the youthful Mars.[41]
"The Martian (SNC) meteorites are critically important for understanding Mars because they provide details of petrography and chemistry that cannot (yet) be measured in situ, and they provide ‘‘ground truths’’ for spectral analyses from the Martian surface using Mo¨ssbauer, thermal emission, and visible, near-IR, and mid-IR reflectance techniques. The recent discovery of a new 815 g Martian meteorite in the Miller Range of Antarctica [Satterwhite and Righter, 2004] provides us with a new sample with which to test hypotheses developed in studies of other nakhlite samples."[42]
The image third down on the right is of the Mackinac Island meteorite, discovered on Mars by the NASA Opportunity rover on October 13, 2009.
At top left is "the first meteorite of any type ever identified on another planet. The pitted, basketball-size object is mostly made of iron and nickel. Readings from spectrometers on the rover determined that composition. Opportunity used its panoramic camera to take the images used in this approximately true-color composite on the rover's 339th martian day, or sol (Jan. 6, 2005). This composite combines images taken through the panoramic camera's 600-nanometer (red), 530-nanometer (green), and 480-nanometer (blue) filters."[43]
Comparison of the two meteorites shown here suggests that the left one is a much more recent fall.
Heliospheres
"In situ measurements in the inner heliosphere (~ 1 AU) indicate that alpha particles can be accelerated up to the local Alfvén speed in the proton frame (Marsch et al. 1982; Neugebauer et al. 1994; Bourouaine et al. 2011a, 2011b). However, the differential speed between alpha particles and protons rarely exceeds the local Alfvén speed, because super-Alfvénic alpha particle beams lead to the excitation of [Alfvén/ion-cyclotron] A/IC and [fast-magnetosonic/whistler] FM/W waves (Li & Habbal 2000; Gary et al. 2000; Verscharen & Chandran 2013), and the amplified waves can decelerate the alpha particles (Kaghashvili et al. 2004; Lu et al. 2009)."[44]
Stellar surface fusion
"Concerning the particles which interact at the Sun, evidence for accelerated 3He enrichment was obtained from the detection (Share & Murphy 1998) of a gamma-ray line at 0.937 MeV produced by the reaction 16O(3He,p)18F∗".[45]
For "essentially all of [some 20] flares 3He/4He can be as large as 0.1, while for some of them values as high as 1 are possible. In addition, [...] for the particles that interact and produce gamma rays, 3He enrichments are present for both impulsive and gradual flares."[45]
"Plasma is the medium for magnetically or inertially-confined controlled thermonuclear fusion. A plasma of deuterium and tritium ions heated to a temperature of 108 degrees Kelvin undergoes thermonuclear burn, producing energetic helium ions and neutrons from fusion reactions."[46]
On the right is a diagram illustrating deuterium-tritium fusion in three steps:
- the D and T accelerating towards each other at thermonuclear speeds,
- the creation of an unstable He-5 nucleus,
- the ejection of a neutron and repulsion of the He-4 nucleus.
The "energy" is released in the form of the velocities of the constituent parts being thrown apart, or radiated apart.
Dose equivalents
Def. the dose received in one hour at a distance of 1 cm from a point source of 1 mg of radium in a 0.5 mm thick platinum enclosure is called a sievert.
Equivalent dose to a tissue is found by multiplying the absorbed dose, in gray, by a weighting factor (WR). The relation between absorbed dose D and equivalent dose H is thus:
- <math>H = W_R \cdot D</math>.
The weighting factor (sometimes referred to as a quality factor) is determined by the radiation type and energy range.[47]
- <math>H_T = \sum_R W_R \cdot D_{T,R}\ ,</math>
where
- HT is the equivalent dose absorbed by tissue T
- DT,R is the absorbed dose in tissue T by radiation type R
- WR is the weighting factor defined by the following table
| Radiation type and energy | WR | |
|---|---|---|
| electrons, muons, photons (all energies) | 1 | |
| protons and charged pions | 2 | |
| alpha particles, fission fragments, heavy ions | 20 | |
| neutrons (function of linear energy transfer L in keV/μm) |
L < 10 | 1 |
| 10 ≤ L ≤ 100 | 0.32·L − 2.2 | |
| L > 100 | 300 / sqrt(L) | |
Thus for example, an absorbed dose of 1 Gy by alpha particles will lead to an equivalent dose of 20 Sv. The maximum weight of 30 is obtained for neutrons with L = 100 keV/μm.
Alpha radiation is an average of about 20 times more dangerous, and in experiments with inhaled alpha emitters, up to 1000 times more dangerous[2] than an equivalent activity of beta emitting or gamma emitting radioisotopes.
In the image on the right is a comparison of alpha and beta radiation effects. The primary advantage of alpha particle (α) emitters over other types of radioactive sources is their very high linear energy transfer (LET) and relative biological effectiveness (RBE).[48] Beta particle (β) emitters such as Yttrium-90 can travel considerable distances beyond the immediate tissue before depositing their energy, while alpha particles deposit their energy in 70–100 μm long tracks.[49]
The favorable geometric situation for α-particles in small-scale metastases (e.g., in the adjuvant setting) is depicted in a scanning electron microscopy micrograph of micro-metastatic clusters from ovarian cancer on the peritoneal lining (mouse). The range of the α-particles in red (here ~50–70 μm), can hardly reach the surrounding normal healthy cells other than possibly the mesothelium and its sub-layer. They cannot reach the epithelial cells of the intestinal lining. The situation for β−particles on the other hand, shows that a great deal of its energy will be deposited far away from the binding site and possibly into healthy tissue as demonstrated by the white dashed line (here ~700 μm). Consequently, it may add to side effects. Bar equals 100 μm.
Alpha particle X-ray spectrometer
Some of the alpha particles are absorbed by the atomic nuclei. The [alpha,proton] process produces protons of a defined energy which are detected. Sodium, magnesium, silicon, aluminium and sulfur can be detected by this method. This method was only used in the Mars Pathfinder APXS.
"The Alpha Particle X-ray Spectrometer on the [Mars] Opportunity rover determined major and minor elements of soils and rocks in Meridiani Planum. Chemical compositions differentiate between basaltic rocks, evaporite-rich rocks, basaltic soils, and hematite-rich soils. Although soils are compositionally similar to those at previous landing sites, differences in iron and some minor element concentrations signify the addition of local components. Rocky outcrops are rich in sulfur and variably enriched in bromine relative to chlorine. The interaction with water in the past is indicated by the chemical features in rocks and soils at this site."[50]
Alpha-particle spectroscopy
{{free media}}On the right is a simulated spectrum containing from left to right the peaks due to 209Po, 210Po, 239Pu and 241Am. The fact that isotopes such as 239Pu and 241Am have more than one alpha line indicates that the nucleus has the ability to be in different discrete energy levels.
"Calibration: [Multichannel Analyzer] MCA does not work on energy, it works on voltage. To relate the energy to voltage one must calibrate the detection system. Here different alpha emitting sources of known energy were placed under the detector and the full energy peak is recorded."[51]
Cadmium telluride radiation detectors
Cadmium telluride (CdTe) doped with chlorine is used as a radiation detector for [X-rays], gamma rays, beta particles and alpha particles. CdTe can operate at room temperature allowing the construction of compact detectors for a wide variety of applications in nuclear spectroscopy.[52] The properties that make CdTe superior for the realization of high performance gamma- and x-ray detectors are high atomic number, large bandgap and high electron mobility ~1100 cm2/V·s, which result in high intrinsic μτ (mobility-lifetime) product and therefore high degree of charge collection and excellent spectral resolution.
Heavy ion telescopes
"The higher energy proton detector [on Explorer 45] is a telescope detector system consisting of two surface barrier solid-state detectors behind a 2.2-kilogauss magnet used to sweep out electrons of energy less than 300 keV."[53] "This telescope measured the flux of protons in six channels covering the energy range 24.3 to 300 keV."[54]
"The heavy ion telescope [Explorer 45] had detectors of thicknesses 3.4 and 100 micrometers. This telescope uniquely identified the presence of protons, alpha particles (Z=2), and two groups of heavier ions, (Li,Be,B) and (C,N,O), plus ions with Z>=9. The heavy ion telescope measured proton fluxes in six channels covering the energy range 365 to 872 keV, and the fluxes of alpha particles in the energy ranges 1.16 to 1.74 keV and 1.74 to 3.15 keV. It measured the fluxes of Li, Be, and B ions in the ranges 3.6 to 7.1 MeV, 6.1 to 9.7 MeV, and 8.7 to 12.2 MeV, respectively, and the fluxes of C, N, and O ions in the ranges 12.1 to 15.7 MeV, 15.6 to 19.2 MeV, and 19.1 to 22.7 MeV, respectively. And it measured the flux of Z>=9 ions with energies > 20 MeV."[54]
Cloud chambers
The cloud chamber, also known as the Wilson chamber, is a particle detector used for detecting ionizing radiation. In its most basic form, a cloud chamber is a sealed environment containing a supersaturated vapor of water or alcohol. When a charged particle (for example, an alpha or beta particle) interacts with the mixture, it ionizes it. The resulting ions act as condensation nuclei, around which a mist will form (because the mixture is on the point of condensation). The high energies of alpha and beta particles mean that a trail is left, due to many ions being produced along the path of the charged particle. These tracks have distinctive shapes (for example, an alpha particle's track is broad and shows more evidence of deflection by collisions, while an electron's is thinner and straight). When any uniform magnetic field is applied across the cloud chamber, positively and negatively charged particles will curve in opposite directions, according to the Lorentz force law with two particles of opposite charge.
The diffusion cloud chamber differs from the expansion cloud chamber in that it is continuously sensitized to radiation, and in that the bottom must be cooled to a rather low temperature, generally as cold as -15 degrees fahrenheit.[55] Alcohol vapor is also often used due to its different phase transition temperatures. Dry-ice-cooled cloud chambers are a common demonstration and hobbyist device; the most common fluid used in them is isopropyl alcohol, though methyl alcohol can be encountered as well. There are also water-cooled diffusion cloud chambers, using ethylene glycol.
"The bubble chamber reveals the tracks of subatomic particles as trails of bubbles in a superheated liquid, usually liquid hydrogen.[56] Bubble chambers can be made physically larger than cloud chambers, and since they are filled with much-denser liquid material, they reveal the tracks of much more energetic particles.
Alpha-Scattering Surface Analyzer
{{free media}}{{free media}}Lunar soil surveys by an alpha-scattering instrument onboard Surveyor 6 were completed using photographic and alpha particle backscattering methods, similar instrument, the APXS, was used onboard several Mars missions.[57]
The alpha-scattering surface analyzer was designed to measure directly the abundances of the major elements of the lunar surface. The instrumentation consisted of an alpha source (curium 242) collimated to irradiate a 100 mm (3.94 in) diameter opening in the bottom of the instrument where the sample was located and two parallel but independent charged particle detector systems. One system, containing two sensors, detected the energy spectra of the alpha particles scattered from the lunar surface, and the other, containing four sensors, detected energy spectra of the protons produced via reactions (alpha and protons) in the surface material. Each detector assembly was connected to a pulse height analyzer.
A digital electronics package, located in a compartment on the spacecraft, continuously telemetered signals to Earth whenever the experiment was operating. The spectra contained quantitative information on all major elements in the samples except for hydrogen, helium, and lithium. Curium collected on the collimator films and was scattered by the gold plating on the inside bottom of the sensor head. This resulted in a gradually increasing background and reduction of the sensitivity technique for heavy elements. One proton detector was turned off during the second day of operation because of noise. A total of 43 hours of data was obtained from November 11 to November 24, 1967. The final data was obtained 4 hours after local sunset. However, after the spacecraft 'hopping' maneuver on November 17, 1967, the sensor head was upside down. Measurements were continued in order to obtain information on solar protons and cosmic rays. Therefore, data for the purpose of the chemical analysis of lunar surface material were obtained only during the first 30 hours of operation. During this period, 27 hours and 44 min of data were known to be noise free.
Kiel Electron Telescope
{{fairuse}}"The Kiel Electron Telescope (KET) on-board Ulysses measures proton and alpha-particles in the energy range from 5 MeV/n to >2 GeV/n."[58]
The "26-day averaged “quiet time” count rates of >2 GeV protons and >2 GeV/n alpha-particles [are] from Ulysses’ launch in 1990 to mid 2002."[59]
With respect to the graphs on the right, the "evolution of the maximum latitudinal extent of the heliospheric current sheet α (a) and the solar polar magnetic field strength for the Southern and Northern Hemisphere (b) are displayed [...], together with the daily averaged count rate of 100– 125 MeV protons (c) and the 26-day averaged “quiet time” count rates of >2 GeV protons and >2 GeV/n alpha-particles (d) from Ulysses’ launch in 1990 to mid 2002. In panel (b) the corresponding 20 nHz smoothed solar polar magnetic field strength is superimposed. The 20 nHz low pass filter is used by the Wilcox Solar Observatory to eliminate yearly geometric projection effects. From the time profiles it follows that the two hemispheres reversed their polarities in 1990 and 2000. Hence, the heliospheric magnetic field is expected to reverse its polarity accordingly. In 2001 the northern polar coronal hole was formed (McComas et al., 2001a), showing the corresponding signatures in the heliospheric magnetic field (Smith et al., 2001), indicating the decline towards solar minimum. It is important to note that such interplanetary signatures have not been observed by Ulysses in 2000, when the spacecraft was at 80° S heliographic latitude."[59]
"The 26-day averaged “quiet time” counting rates in panel (d) of [the graphs] are presented as percentage changes with respect to the maximal rates Cmax measured in mid 1997 at solar minimum. “Quiet time” profiles have been determined by using only time periods when the 100–125 MeV proton channel (panel (c) of [the graphs]) showed no contribution of solar or interplanetary particles (Heber et al., 1999). Marked by shading in (c) and (d) are the Jovian flyby in 1992 (JE), the two rapid pole to pole passages in 1994/1995 and 2000/2001 (FLS), and the ecliptic crossing in 1998 (EC). The observed variations in the particle intensities are caused by temporal and spatial variations due to the Ulysses trajectory. Therefore, the variation from solar minimum to solar maximum in 2000 does not reflect the total modulation amplitude at these rigidities. The two rapid pole to pole transitions at ∼1.5 AU should provide the best “snapshot” of the spatial distribution of cosmic rays in the inner heliosphere at solar minimum and maximum, respectively."[59]
"Although numerous solar particle events have been observed, the “quiet time” count rates of galactic cosmic ray protons and alpha-particles are continuously increasing during the second fast latitude scan, indicating that none of these events give rise to a modulation barrier, like the March to July activity in 1991 (McDonald et al., 2000)."[59]
Graph (d) "indicates that because of the continuous increase during the rapid pole to pole passage in 2000/2001, no significant latitudinal gradients at solar maximum could be present. If such latitudinal gradients would have been present, then the temporal and Ulysses’ radial variation must have canceled them exactly. We find such a symmetric temporal variation, centered around day 136 of 2001, unlikely, and, therefore, reject this scenario. Since Ulysses moved from a distance of ∼2 AU at southern polar regions inward to 1.34 AU close to the heliographic equator and then back to ∼2 AU over the north pole, a radial gradient of ∼3%/AU would lead to a 1.021 times higher flux at polar regions. A negative latitudinal gradient of the order of 0.026%/degree might be masked by the radial variation."[59]
During "Ulysses’ solar minimum orbit in 1994 to 1997 the cosmic ray observations were dominated by (1) latitudinal, (2) radial, and (3) temporal variations. Therefore, we could determine the latitudinal gradient, during Ulysses’ fast latitude scan, and the radial gradient, when Ulysses was back to the heliographic equator in 1997 (Belov et al., 1999)."[59]
"The cosmic ray time profile, which should not be related to Ulysses’ position, correlates occasionally with the spacecraft distance and/or latitude. Such a correlation can be essentially high on relatively small time intervals (less than a year)."[59]
solar maximum:
- <math>g_r^h</math> = (2.4 ± 0.2)%/AU
- <math>g_{\theta}^h</math> = (−0.01 ± 0.01)%/°
- <math>b_{\delta}^h</math> = 0.93 ± 0.07
solar minimum:
- <math>g_r^h</math> = (0.5 ± 0.2)%/AU
- <math>g_{\theta}^h</math> = (0.12 ± 0.01)%/°
- <math>b_{\delta}^h</math> = n.a.
"Using these parameters [above for solar maximum and minimum] we obtain for protons and alpha- particles correlation coefficients of 0.972 and 0.81. Note that the poor correlation coefficient for the alpha-particles results from the large statistical uncertainties."[59]
"In contrast to solar minimum our analysis indicates a spherically symmetric distribution of cosmic rays around solar maximum. The intensities in the inner heliosphere depend on the radial distance from the Sun only, while in 1994 to 1996 the latitude dependence outside of the streamer belt (∼15°) dominates the observations at solar minimum."[59]
"Since latitudinal gradients were positive at solar minimum in the last cycle and vanishing thereafter, the total modulation is higher at polar latitudes than in the ecliptic. While the observations at solar minimum in an A > 0 solar magnetic cycle confirm the results from advanced modulation models (Potgieter et al., 2001), the distribution obtained by Ulysses during the next A < 0 solar minimum will be a crucial test for such models."[59]
The cosmic ray "mean spatial distribution at solar minimum from 1994 to 1996 is remarkably different from the one at solar maximum from 1998 to mid 2001. While the positive latitudinal gradient dominates the picture at solar minimum this distribution is spherically symmetric around solar maximum, with large radial gradients in the inner heliosphere. The increase in solar activity is accompanied by an increase in the radial gradient. When Ulysses was at high heliographic latitudes above 30° S from mid 1999 on, no significant latitudinal structure could be found until July 2001, when Ulysses was going above ∼50° N and the tilt angle α fell down sharply. It is interesting to note that, as a consequence of the reconstruction of cosmic rays from a latitude dominated to a spherically symmetric distribution at solar maximum, the magnitude of the 11-year cosmic ray cycle is essentially bigger at polar regions than close to the heliospheric equator, particularly near Earth."[59]
"From mid 1999, when Ulysses was above 30° S to mid 2001, a highly variable and slow solar wind has been observed by Ulysses only (McComas et al., 2001b)."[59]
The "expansion described by the tilt angle and structure of the streamer belt to high heliographic latitudes leads to a strong increase in modulation and the form of the cosmic ray spatial distribution in the inner heliosphere. As the tilt angle is decreasing towards solar minimum, with the development since 2001 of the northern polar coronal hole, fast solar wind emanating from that hole has been observed by Ulysses. Nearly simultaneously an increase in the particle intensities at high northern polar latitude can be observed."[59]
IMP-8
{{free media}}"IMP-8 is a NASA/GSFC mission - also known by the names of Explorer 50, and IMP-J (COSPAR designation: 73-078A). IMP-8 is the last in a series of ten IMP missions with the primary objective to perform detailed and near-continuous observations of the Sun/Earth environment (solar wind monitoring, measuring the plasma/field environment of the magnetosheath and the magnetotail). The IMP-8 mission is part of NASA's Sun-Earth Connections research program."[60]
Instruments on-board for measuring alpha particles included the Charged Particle Measurement Experiment (CPME), the Cosmic Ray Nuclei Experiment (CRNE or CHE), and the Energetic Particle Experiment (EPE).[60]
Charged Particle Measurement Experiment
"The [Charged Particle Measurement Experiment] instrument is a solid-state telescope [...] measuring fluxes of protons in 11 energy channels between 0.29 and 140 MeV, and alpha particles in 6 channels between 0.64 and 52 MeV/n. Time resolution for the measurement cycle is 10.24 s."[60]
"The CPME has two major components, the PET (Proton-Electron Telescope) and five thin-window Geiger-Mueller (GM) tubes. The PET measures and identifies electrons, protons, alpha particles, M-nuclei, and Fe-group nuclei. The GM array measures both solar X-rays and the more intense galactic X-ray sources."[60]
"Energetic protons [P] (0.39–440 MeV), alpha particles [α] (0.59–52 MeV/nucleon), and medium nuclei [M] (carbon, nitrogen, and oxygen; 0.7–8.8 MeV/nucleon) have been observed with the Charged Particle Measurement Experiment (CPME) aboard the Interplanetary Monitoring Platform 8 (IMP 8) spacecraft from 1973 to [2004]."[61]
Alpha particle maxima Cycle 20 1700, Cycle 21 2700, Cycle 22 3300, Cycle 23 4100 particles/cm2 s sr.[61]
"Over 27 years the CPME has recorded many events. It was found that not all of the flux channels peaked for the same type of event. The proportion of flux in each energy range is about the same from year to year, but the total flux varies with the solar cycle. Solar cycle 22 had the most extreme changes in flux between solar maximum and solar minimum, while solar cycle 21 saw only moderate changes. Days with maximum integral flux usually occurred in the first half of the solar cycle. Power law relationships between flux and energy were found for all the particle species in the study: protons, alphas, and medium nuclei."[61]
"P/α ratios peaked near solar maximum, while α/M ratios peaked near solar minimum."[61]
Cosmic Ray Nuclei Experiment
"The [Chicago] instrument is also referred to as CRNE (Cosmic Ray Nuclei Experiment). The instrument (mass=7.4 kg) consists of a pair of solid-state telescopes. The main telescope measures nuclei in the energy range of 10 to 100's of MeV/n, and electrons in the range of about 2 to about 25 MeV. The second telescope measures protons and alpha particles in the 0.5-1.8 MeV/n range. The charge resolution improved by using curved detectors."[60]
"To derive radial and latitudinal gradients for >2 GeV/n protons and alpha-particles, data from the Chicago instrument on board IMP-8 and the neutron monitor network have been used to determine the corresponding time profiles at Earth."[59]
Energetic Particle Experiment
"The main detector assembly consists of (i) a three-element telescope (detectors A, B, and C), (ii) a sweeping magnet that keeps low energy electrons (Ee<=200-300 keV) away from the telescope, and (iii) two detectors (D and E) to detect the swept electrons. The telescope covers the proton energy range 50 keV ≤ Ep ≤ 25 MeV and the alpha particle range 2.2 MeV ≤ Ea ≤ 35 MeV. Detectors D and E cover the electron energy range 30 keV ≤ Ee ≤ 200 keV."[60]
Goddard Medium Energy
"The objective was to measure fluxes as a functions of energy and to make elemental identification for protons, alpha particles and heavier ions from < 1 MeV/nucleon to >400 MeV/nucleon as well as to measure the flux of relativistic electrons between 3 and 18 MeV."[60]
"Species identification is performed by the differential energy loss against total energy (dE/dx vs. E) method. The instrument consists of three particle telescopes covering the different energy ranges. Instruments on each IMP are similar, but feature a few key differences, particularly at low energies. The relevant instrument for the relativistic electrons is the Medium Energy Detector (MED) which remained mostly unchanged between spacecraft. Other detectors are used to measure protons and heavy ions, the VLET (Very Low Energy Telescope) and LED (Low Energy Detector). The general structure of any of the IMP particle telescopes is a relatively thin front detector and one or more (thicker) following detectors, each detector capable of quantitative measurement of the energy lost by a particle in it. Collimation and anticoincidence systems are also provided to exclude/distinguish particles entering at steep angles to the detector surfaces and particles of sufficient incident energy to penetrate the detector stack. In the case of the MED, the detectors used are CsI scintillators coupled with photomultiplier tubes[9]."[62]
Proton Electrostatic Analyzer
{{fairuse}}The Proton Electrostatic Analyzer (PESA-L) onboard the Wind satellite "measures the solar wind ions from 3 eV to 30 keV at the [...] 3 s resolution. PESA-L was specifically designed to sample the solar wind protons by tracking the core of their distribution. [...] The on-board proton moment calculation relies on an algorithm that separates the alpha particles from the main proton population."[63]
In the graphs on the right, panel f "shows the difference between the on-board proton temperature (blue line) and the integrated one (black line); the offset between the two temperatures is due to the alpha particles, but one can clearly see the rise in the ion temperature, at the same time as the rise in ion or electron density [panel d], due to the presence of the proton beam."[63]
The "investigated event was not related to an interplanetary coronal mass ejection (ICME). [...] this event is likely a case of reconnection in a kinked open magnetic field line."[63]
"Wind was located relatively far away from the bow shock (∼180 RE) and although the backscattering electrons were observed down to at least 27 eV, the conic was most intense at the energies 65 eV and above."[63]
High-time "resolution particle observations [were] obtained with the EESA-L and PESA-L electrostatic analyzers of the Wind satellite during the solar wind reconnection event on 22 July 1999. Solar wind reconnection is a recently found phenomena (Gosling et al., 2005a) and thus relatively little studied. The time between the observations of the separatrices at Wind was only 56 s and therefore the 3-s burst mode observations of EESA-L and PESA-L as well as Wind 3-s plasma measurements were essential for the identification and analysis of this event. [...] Wind crossed the separatrix and the out-flow regions about a thousand ion skin depths (λi) away from the X-line that is significantly further than the typical crossing distances are in simulations and in most previous in-situ studies. In the Earth’s magnetosphere separatrix regions have been observed to maintain their internal structure several hundreds ion skin depths away from the X-line (Retino ́ et al., 2006; Khotyaintsev et al., 2006), but it is currently unknown how far separatrices can keep their integrity (e.g. Vaivads et al., 2006)."[63]
"The reconnection event was identified by the accelerated plasma flow confined between the bifurcated current sheet. The current sheets bounding the investigated outflow region had the width of 19 λi at the leading boundary and the width of 24 λi at the end boundary. The locations of the separatrices were estimated using an opening angle that is twice the opening angle of the outflow region. In the front separatrix region, a strong proton beam was observed. At the trailing separator boundary, the transition from outside to inside the outflow region was slow-mode like characterized by increases in the proton density and temperature and a decrease in the magnetic field magnitude. Solar wind reconnection events, including this event, typically occur between two quite distinct plasma states (e.g. Gosling et al., 2005a, 2006a; Huttunen et al., 2007) that might account for the observed asymmetries of the separatrix regions on the opposite sides of the outflow region."[63]
"Thermal particle observations revealed several interesting features during the passage of the separatrix and outflow regions: 1) the continuation of solar wind strahl indicating that the field lines Wind crossed were open; 2) ansiotropic elec- tron phase space distributions at energies 18 to 42 eV with 3) electrons flowing towards the X-line in the inner boundary of the outflow region and away from the X-line in the outer boundary of the outflow region and 4) a strong proton beam at the front separatrix region originating from the direction of the X-line."[63]
This "event [...] is the first solar wind reconnection event during which sufficiently high time-resolution observations were available to probe in detail the characteristics of the separatrix and outflow domains."[63]
Acknowledgements
The content on this page was first contributed by: Henry A. Hoff.
Initial content for this page in some instances came from Wikiversity.
See also
References
- ↑ Darling, David. "Alpha particle". Encyclopedia of Science. Archived from the original on 14 December 2010. Retrieved 7 December 2010.
- ↑ 2.0 2.1 Little, John B.; Kennedy, Ann R.; McGandy, Robert B. (1985). "Effect of Dose Rate on the Induction of Experimental Lung Cancer in Hamsters by α Radiation". Radiation Research. 103 (2): 293–9. Bibcode:1985RadR..103..293L. doi:10.2307/3576584. JSTOR 3576584. PMID 4023181.
- ↑ Grellier, James; et al. (2017). "Risk of lung cancer mortality in nuclear workers from internal exposure to alpha particle-emitting radionuclides". Epidemiology. 28 (5): 675–684. doi:10.1097/EDE.0000000000000684. PMC 5540354. PMID 28520643.
- ↑ Radford, Edward P.; Hunt, Vilma R. (1964). "Polonium-210: A Volatile Radioelement in Cigarettes". Science. 143 (3603): 247–249. Bibcode:1964Sci...143..247R. doi:10.1126/science.143.3603.247. PMID 14078362.
- ↑ Cowell, Alan (24 November 2006). "Radiation Poisoning Killed Ex-Russian Spy". The New York Times. Retrieved 15 September 2011.
- ↑ Magazine "nuclear energy" (III/18 (203) special edition, Volume 10, Issue 2 /1967.
- ↑ SemperBlotto (16 February 2007). "alpha particle". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 July 2019.
- ↑ SemperBlotto (19 February 2005). "alpha particle". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 July 2019.
- ↑ J. R. Comfort, J. F. Decker, E. T. Lynk, M. O. Scully, and A. R. Quinton (1966). "Energy loss and straggling of alpha particles in metal foils". Physical Review. 150 (1): 249. doi:10.1103/PhysRev.150.249. Retrieved 2016-01-29. Unknown parameter
|month=ignored (help) - ↑ http://library.thinkquest.org/27954/dequ.htm
- ↑ http://chemteam.info/Radioactivity/Writing-Alpha-Beta.html
- ↑ Loveland, W., Morrissey, D. J., Seaborg, G. T., Modern Nuclear Chemistry, 2006, John Wiley & Sons, 221.
- ↑ 13.0 13.1 13.2 13.3 13.4 Geiger, Hans; Marsden, Ernest (1909). "On a Diffuse Reflection of the α-Particles" (PDF). Proceedings of the Royal Society of London A. 82 (557): 495–500. Bibcode:1909RSPSA..82..495G. doi:10.1098/rspa.1909.0054.
- ↑ 14.0 14.1 14.2 14.3 Geiger, Hans (1908). "On the Scattering of α-Particles by Matter" (PDF). Proceedings of the Royal Society of London A. 81 (546): 174–177. Bibcode:1908RSPSA..81..174G. doi:10.1098/rspa.1908.0067.
- ↑ 15.0 15.1 15.2 "Rutherford's Nuclear World: The Story of the Discovery of the Nucleus". American Institute of Physics. Retrieved 2014-10-23.
- ↑ 16.0 16.1 16.2 Geiger, Hans (1910). "The Scattering of the α-Particles by Matter" (PDF). Proceedings of the Royal Society of London A. 83 (565): 492–504. Bibcode:1910RSPSA..83..492G. doi:10.1098/rspa.1910.0038.
- ↑ 17.0 17.1 17.2 17.3 Rutherford, Ernest (1911). "The Scattering of α and β Particles by Matter and the Structure of the Atom". Philosophical Magazine. Series 6. 21 (125): 669–688. doi:10.1080/14786440508637080.
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 18.11 18.12 Geiger, Hans; Marsden, Ernest (1913). "The Laws of Deflexion of α Particles through Large Angles" (PDF). Philosophical Magazine. Series 6. 25 (148): 604–623. doi:10.1080/14786440408634197.
- ↑ 19.0 19.1 19.2 19.3 19.4 S. Biswas & C. E. Fichtel (1965). "Composition of Solar Cosmic Rays". Space Science Reviews. 4 (5–6): 709–736. Bibcode:1965SSRv....4..709B. doi:10.1007/BF00216274. Retrieved 2018-5-23. Unknown parameter
|month=ignored (help); Check date values in:|accessdate=(help) - ↑ 20.0 20.1 20.2 np (6 November 1996). Systematics Alpha decay. uct.ac.za: Physics Department. p. 1. Retrieved 2018-04-01.
- ↑ 21.0 21.1 Krane, K. S. (1988). Introductory Nuclear Physics. John Wiley & Sons. p. 537. ISBN 0-471-80553-X.
- ↑ 22.0 22.1 Appenzeller; Harwit; Kippenhahn; Strittmatter; Trimble, eds. (1998). Astrophysics Library (3rd ed.). New York: Springer.
- ↑ 23.0 23.1 Carroll, Bradley W. & Ostlie, Dale A. (2007). An Introduction to Modern Stellar Astrophysics. Addison Wesley, San Francisco. ISBN 978-0-8053-0348-3.
- ↑ John Avison (November 2014). The World of Physics. Nelson Thornes. pp. 397–. ISBN 978-0-17-438733-6.
- ↑ Audi, Georges; Kondev, Filip G.; Wang, Meng; Huang, Wen Jia; Naimi, Sarah (2017), "The NUBASE2016 evaluation of nuclear properties" (PDF), Chinese Physics C, 41 (3): 030001–1—030001–138, Bibcode:2017ChPhC..41c0001A, doi:10.1088/1674-1137/41/3/030001
- ↑ Agakishiev, H.; et al. (STAR collaboration) (2011). "Observation of the antimatter helium-4 nucleus". Nature. 473 (7347): 353–6. arXiv:1103.3312. Bibcode:2011Natur.473..353S. doi:10.1038/nature10079. PMID 21516103.. See also "Erratum". Nature. 475 (7356): 412. 2011. arXiv:1103.3312. doi:10.1038/nature10264.
- ↑ "Antihelium-4: Physicists nab new record for heaviest antimatter". PhysOrg. 24 April 2011. Retrieved 15 November 2011.
- ↑ Jayant V. Narlikar (1995). From Black Clouds to Black Holes. World Scientific. ISBN 9810220332.
- ↑ Lalovic, M.; Werle, H. (1970). "The energy distribution of antimonyberyllium photoneutrons". Journal of Nuclear Energy. 24 (3): 123–132. Bibcode:1970JNuE...24..123L. doi:10.1016/0022-3107(70)90058-4.
- ↑ Ahmed, S. N. (2007). Physics and Engineering of Radiation Detection. p. 51. Bibcode:2007perd.book.....A. ISBN 978-0-12-045581-2.
- ↑ Handbook on Photonuclear Data for Applications: Cross-sections and Spectra. IAEA.
- ↑ Sean G. Ryan, Andrew J. Norton (2010). Stellar Evolution and Nucleosynthesis. Cambridge University Press. p. 135. ISBN 978-0-521-13320-3.
- ↑ W. H. Camiel, C. Doom de Loore (1992). Camiel W. H. de Loore, ed. Structure and evolution of single and binary stars, In: Volume 179 of Astrophysics and space science library. Springer. pp. 95–97. ISBN 978-0-7923-1768-5.
- ↑ 34.0 34.1 Principles and Perspectives in Cosmochemistry, Springer, 2010, ISBN 9783642103681, page 233
- ↑ Neutron Induced Single Event Upset Dependence on Bias Voltage for CMOS SRAM With BPSG
- ↑ An Accurate and Comprehensive Soft Error Simulator Y. Tosaka, S. Satoh, and H. Oka - Fujitsu Laboratories
- ↑ 37.0 37.1 37.2 Paul J. Bjorkholm, Leon Golub, an Paul Gorenstein (1973). "Distribution of 222Rn and 210Po on the lunar surface as observed by the alpha particle spectrometer". Geochimica et Cosmochimica Acta. 3: 2793–802. Bibcode:1973LPSC....4.2793B. Retrieved 7 July 2019.
- ↑ 38.0 38.1 38.2 Norman F. Ness (16 May 1968). Lunar Explorer 35, In: XIth COSPAR (PDF). Greenbelt, Maryland USA: NASA-Goddard Space Flight Center. pp. 1–32. Retrieved 9 July 2019.
- ↑ Opportunity Rover Finds an Iron Meteorite on Mars. JPL. January 19, 2005. Retrieved 2006-12-12.
- ↑ 40.0 40.1 Sue Lavoie (August 6, 2009). PIA12193: 'Block Island' Meteorite on Mars, Sol 1961 (False Color). Pasadena, California USA: NASA/JPL. Retrieved 2013-05-30.
- ↑ 41.0 41.1 Steve Jurvetson (December 21, 2012). It came from Mars. flickr from Yahoo!. Retrieved 2013-02-24.
- ↑ M. Darby Dyar, Allan H. Treiman, Carlé M. Pieters, Takahiro Hiroi, Melissa D. Lane, and Vanessa O’Connor (September). "MIL03346, the most oxidized Martian meteorite: A first look at spectroscopy, petrography, and mineral chemistry" (PDF). Journal of Geophysical Research: Planets (1991–2012). 110 (E9): 5. doi:10.1029/2005JE002426. Retrieved 2014-01-10. Check date values in:
|date=, |year= / |date= mismatch(help) - ↑ NASA (January 19, 2005). PIA07269: Iron Meteorite on Mars. Pasadena, California USA: NASA. Retrieved 2013-02-16.
- ↑ Sofiane Bourouaine, Daniel Verscharen, Benjamin D. G. Chandran, Bennett A. Maruca, and Justin C. Kasper (9 October 2013). "Limits on alpha particle temperature anisotropy and differential flow from kinetic instabilities: Solar wind observations". The Astrophysical Journal Letters. 777 (1): 1–5. arXiv:1309.4010. Retrieved 7 July 2019.
- ↑ 45.0 45.1 Reuven Ramaty, Vincent Tatischeff, J. P. Thibaud, Benzion Kozlovsky, and Natalie Mandzhavidze (2000). "6Li from Solar Flares". The Astrophysical Journal. 534 (2): L207–10. arXiv:astro-ph/0003356. Bibcode:2000ApJ...534L.207R. doi:10.1086/312671. Retrieved 2014-04-08. Unknown parameter
|month=ignored (help) - ↑ CK Birdsall, A. Bruce Langdon (October 1, 2004). Plasma Physics via Computer Simulation. New York: CRC Press. p. 479. ISBN 0-7503-1035-1 Check
|isbn=value: checksum (help). Retrieved 2011-12-17. - ↑ The 2007 Recommendations (PDF). International Commission on Radiological Protection. Retrieved 2011-04-15.
- ↑ Kane, Suzanne Amador (2003). Introduction to physics in modern medicine (Repr. ed.). London: Taylor & Francis. p. 243. ISBN 9780415299633.
- ↑ Elgqvist, Jörgen; Frost, Sofia; Pouget, Jean-Pierre; Albertsson, Per (2014). "The Potential and Hurdles of Targeted Alpha Therapy – Clinical Trials and Beyond". Frontiers in Oncology. 3: 324. doi:10.3389/fonc.2013.00324. PMC 3890691. PMID 24459634.
- ↑ R. Rieder, R. Gellert, R. C. Anderson, J. Brückner, B. C. Clark, G. Dreibus, T. Economou, G. Klingelhöfer, G. W. Lugmair, D. W. Ming, S. W. Squyres, C. d'Uston, H. Wänke1, A. Yen, J. Zipfel (3 December 2004). "Chemistry of Rocks and Soils at Meridiani Planum from the Alpha Particle X-ray Spectrometer". Science. 306 (5702): 1745–9. Retrieved 7 July 2019.
- ↑ Vsmith (6 February 2015). "Alpha-particle spectroscopy". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 7 July 2019.
- ↑ P. Capper (1994). Properties of Narrow-Gap Cadmium-Based Compounds. London, UK: INSPEC, IEE. ISBN 0-85296-880-9.
- ↑ D. J. Williams, T. A. Fritz, A. Konradi (1973). "Observations of proton spectra (1.0≤ Ep≤ 300 keV) and fluxes at the plasmapause". Journal of Geophysical Research. 78 (22): 4751–5. Bibcode:1973JGR....78.4751W. doi:10.1029/JA078i022p04751. Retrieved 2013-06-22. Unknown parameter
|month=ignored (help) - ↑ 54.0 54.1 Theodore Allan Fritz (June 14, 2013). S-Cubed A. Greenbelt, Maryland USA: NASA Goddard Space Flight Center. Retrieved 2013-06-22.
- ↑ Frisch, O.R. (2013-10-22). Progress in Nuclear Physics, Band 3. p. 1. ISBN 9781483224923.
- ↑ Donald A. Glaser (1952). "Some Effects of Ionizing Radiation on the Formation of Bubbles in Liquids". Physical Review. 87 (4): 665. Bibcode:1952PhRv...87..665G. doi:10.1103/PhysRev.87.665.
- ↑ NASA, JPL,. "Alpha Particle X-Ray Spectrometer (APXS) – Mars Science Laboratory". mars.nasa.gov. Retrieved 2016-11-25.
- ↑ A. V. Belov, E. A. Eroshenko, B. Heber, V. G. Yanke, A. Raviart, K. Röhrs, R, Müller-Mellin, H. Kunow, G. Wibberenz, and C. Paizis (15 August 2001). "Latitudinal and radial variation of >2 GeV/n protons and alpha particles in the southern heliosphere at solar maximum: ULYSSES COSPIN/KET and neutron monitor network observations". Proceedings of the 27th International Cosmic Ray Conference. 2001: 3996–9. Bibcode:2001ICRC...10.3996B.
|access-date=requires|url=(help) - ↑ 59.00 59.01 59.02 59.03 59.04 59.05 59.06 59.07 59.08 59.09 59.10 59.11 59.12 59.13 A. V. Belov, E. A. Eroshenko, B. Heber, V. G. Yanke, A. Raviart, R. Müller-Mellin, H. Kunow (14 February 2003). "Latitudinal and radial variation of >2 GeV/n protons and alpha-particles at solar maximum: ULYSSES COSPIN/KET and neutron monitor network observations". Annales Geophysicae. 21 (6): 1295–1302. Retrieved 8 July 2019.
- ↑ 60.0 60.1 60.2 60.3 60.4 60.5 60.6 "IMP-8". EO Portal. Retrieved 2018-06-19.
- ↑ 61.0 61.1 61.2 61.3 K. D. C. Simunac and T. P. Armstrong (October 2004). "Solar cycle variations in solar and interplanetary ions observed with Interplanetary Monitoring Platform 8". Journal of Geophysical Research. 109 (A10). doi:10.1029/2003JA010194. Retrieved 9 July 2019.
- ↑ B. Taylor, G. Vacanti, E. Maddox, C. I. Underwood (December 2011). "The Interplanetary Electron Model (IEM)" (PDF). IEEE Transactions on Nuclear Science. 58 (6): 2785–2792. doi:10.1109/TNS.2011.2171718. Retrieved 9 July 2019.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 63.7 K. E. J. Huttunen, S. D. Bale, and C. Salem (12 September 2008). "Wind observations of low energy particles within a solar wind reconnection region" (PDF). Annales Geophysicae. 26: 2701–10. Retrieved 9 July 2019.
External links
Template:Principles of radiation astronomy{{Radiation astronomy resources}}Template:Sisterlinks