Abacavir clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Studies
Adults
Therapy-Naive Adults
CNA30024 was a multicenter, double-blind, controlled trial in which 649 HIV-1-infected, therapy-naive adults were randomized and received either ZIAGEN (300 mg twice daily), lamivudine (150 mg twice daily), and efavirenz (600 mg once daily); or zidovudine (300 mg twice daily), lamivudine (150 mg twice daily), and efavirenz (600 mg once daily). The duration of double-blind treatment was at least 48 weeks. Trial participants were male (81%), Caucasian (51%), black (21%), and Hispanic (26%). The median age was 35 years; the median pretreatment CD4+ cell count was 264 cells/mm3, and median plasma HIV-1 RNA was 4.79 log10 copies/mL. The outcomes of randomized treatment are provided in Table 7.
 |
After 48 weeks of therapy, the median CD4+ cell count increases from baseline were 209 cells/mm3 in the group receiving ZIAGEN and 155 cells/mm3 in the zidovudine group. Through Week 48, 8 subjects (2%) in the group receiving ZIAGEN (5 CDC classification C events and 3 deaths) and 5 subjects (2%) on the zidovudine arm (3 CDC classification C events and 2 deaths) experienced clinical disease progression. CNA3005 was a multicenter, double-blind, controlled trial in which 562 HIV-1-infected, therapy-naive adults were randomized to receive either ZIAGEN (300 mg twice daily) plus COMBIVIR® (lamivudine 150 mg/zidovudine 300 mg twice daily), or indinavir (800 mg 3 times a day) plus COMBIVIR twice daily. The trial was stratified at randomization by pre-entry plasma HIV-1 RNA 10,000 to 100,000 copies/mL and plasma HIV-1 RNA greater than 100,000 copies/mL. Trial participants were male (87%), Caucasian (73%), black (15%), and Hispanic (9%). At baseline the median age was 36 years; the median baseline CD4+ cell count was 360 cells/mm3, and median baseline plasma HIV-1 RNA was 4.8 log10 copies/mL. Proportions of subjects with plasma HIV-1 RNA less than 400 copies/mL (using Roche AMPLICOR HIV-1 MONITOR Test) through 48 weeks of treatment are summarized in Table 8
 |
Treatment response by plasma HIV-1 RNA strata is shown in Table 9.
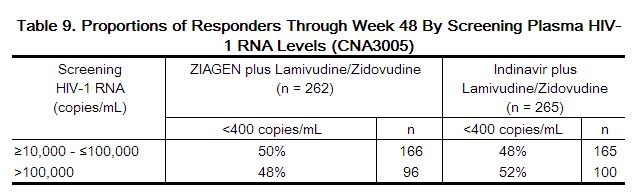 |
In subjects with baseline viral load greater than 100,000 copies/mL, percentages of subjects with HIV-1 RNA levels less than 50 copies/mL were 31% in the group receiving abacavir versus 45% in the group receiving indinavir.
Through Week 48, an overall mean increase in CD4+ cell count of about 150 cells/mm3 was observed in both treatment arms. Through Week 48, 9 subjects (3.4%) in the group receiving abacavir sulfate (6 CDC classification C events and 3 deaths) and 3 subjects (1.5%) in the group receiving indinavir (2 CDC classification C events and 1 death) experienced clinical disease progression.
CNA30021 was an international, multicenter, double-blind, controlled trial in which 770 HIV-1-infected, therapy-naive adults were randomized and received either abacavir 600 mg once daily or abacavir 300 mg twice daily, both in combination with lamivudine 300 mg once daily and efavirenz 600 mg once daily. The double-blind treatment duration was at least 48 weeks. Trial participants had a mean age of 37 years; were male (81%), Caucasian (54%), black (27%), and American Hispanic (15%). The median baseline CD4+ cell count was 262 cells/mm3 (range 21 to 918 cells/mm3) and the median baseline plasma HIV-1 RNA was 4.89 log10 copies/mL (range: 2.60 to 6.99 log10 copies/mL).
The outcomes of randomized treatment are provided in Table 10.
 |
After 48 weeks of therapy, the median CD4+ cell count increases from baseline were 188 cells/mm3 in the group receiving abacavir 600 mg once daily and 200 cells/mm3 in the group receiving abacavir 300 mg twice daily. Through Week 48, 6 subjects (2%) in the group receiving ZIAGEN 600 mg once daily (4 CDC classification C events and 2 deaths) and 10 subjects (3%) in the group receiving ZIAGEN 300 mg twice daily (7 CDC classification C events and 3 deaths) experienced clinical disease progression. None of the deaths were attributed to trial medications.
Pediatric Trials
Therapy-Experienced Pediatric Subjects
CNA3006 was a randomized, double-blind trial comparing ZIAGEN 8 mg/kg twice daily plus lamivudine 4 mg/kg twice daily plus zidovudine 180 mg/m2 twice daily versus lamivudine 4 mg/kg twice daily plus zidovudine 180 mg/m2 twice daily. Two hundred and five therapy-experienced pediatric subjects were enrolled: female (56%), Caucasian (17%), black (50%), Hispanic (30%), median age of 5.4 years, baseline CD4+ cell percent greater than 15% (median = 27%), and median baseline plasma HIV-1 RNA of 4.6 log10 copies/mL. Eighty percent and 55% of subjects had prior therapy with zidovudine and lamivudine, respectively, most often in combination. The median duration of prior nucleoside analogue therapy was 2 years. At 16 weeks the proportion of subjects responding based on plasma HIV-1 RNA less than or equal to 400 copies/mL was significantly higher in subjects receiving ZIAGEN plus lamivudine plus zidovudine compared with subjects receiving lamivudine plus zidovudine, 13% versus 2%, respectively. Median plasma HIV-1 RNA changes from baseline were -0.53 log10 copies/mL in the group receiving ZIAGEN plus lamivudine plus zidovudine compared with -0.21 log10 copies/mL in the group receiving lamivudine plus zidovudine. Median CD4+ cell count increases from baseline were 69 cells/mm3 in the group receiving ZIAGEN plus lamivudine plus zidovudine and 9 cells/mm3 in the group receiving lamivudine plus zidovudine. .[1]
References
- ↑ "ZIAGEN (ABACAVIR SULFATE) TABLET, FILM COATED ZIAGEN (ABACAVIR SULFATE) SOLUTION [VIIV HEALTHCARE COMPANY]". Retrieved 30 December 2013.
Adapted from the FDA Package Insert.