Wilson's disease pathophysiology: Difference between revisions
| Line 13: | Line 13: | ||
[[Image:Copper metabolism.png|center|framed|Normal absorption and distribution of copper. Cu = copper, CP = ceruloplasmin, green = ATP7B carrying copper.]] | [[Image:Copper metabolism.png|center|framed|Normal absorption and distribution of copper. Cu = copper, CP = ceruloplasmin, green = ATP7B carrying copper.]] | ||
It is not clear why Wilson's disease causes hemolysis, but various lines of evidence suggest that high levels of free (non-ceruloplasmin bound) copper have a direct effect on either oxidation of [[hemoglobin]], inhibition of energy-supplying enzymes in the [[red blood cell]], or direct damage to the [[cell membrane]].<ref>{{cite book |last=Lee |first=GR |editor=Lee GR, Foerster J, Lukens J ''et al'' |title=Wintrobe's clinical hematology |edition=10th |volume=vol 1 |year=1999 |publisher=Williams & Wilkins|isbn=0-683-18242-0 |pages=1298 |chapter=Chapter 48: acquired hemolytic anaemias resulting from direct effects of infectious, chemical or physical agents }}</ref> | It is not clear why Wilson's disease causes hemolysis, but various lines of evidence suggest that high levels of free (non-ceruloplasmin bound) copper have a direct effect on either oxidation of [[hemoglobin]], inhibition of energy-supplying enzymes in the [[red blood cell]], or direct damage to the [[cell membrane]].<ref>{{cite book |last=Lee |first=GR |editor=Lee GR, Foerster J, Lukens J ''et al'' |title=Wintrobe's clinical hematology |edition=10th |volume=vol 1 |year=1999 |publisher=Williams & Wilkins|isbn=0-683-18242-0 |pages=1298 |chapter=Chapter 48: acquired hemolytic anaemias resulting from direct effects of infectious, chemical or physical agents }}</ref> | ||
Revision as of 19:41, 22 August 2012
|
Wilson's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Wilson's disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Wilson's disease pathophysiology |
|
Risk calculators and risk factors for Wilson's disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pathophysiology

Copper is needed by the body for a number of functions, predominantly as a cofactor for a number of enzymes such as ceruloplasmin, cytochrome c oxidase, dopamine β-hydroxylase, superoxide dismutase and tyrosinase.
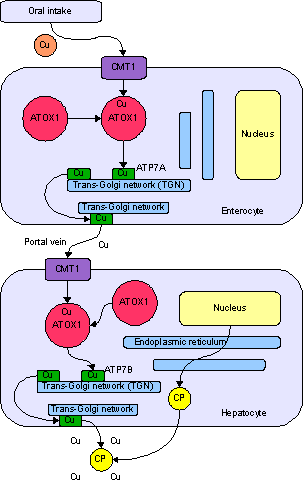
Copper enters the body through the digestive tract. A transporter protein on the cells of the small bowel, copper membrane transporter 1 (CMT1), carries copper inside the cells, where some is bound tometallothionein and part is carried by ATOX1 to an organelle known as the trans-Golgi network. Here, in response to rising concentrations of copper, an enzyme called ATP7A releases copper into the portal vein to the liver. Liver cells also carry the CMT1 protein, and metallothionein and ATOX1 bind it inside the cell, but here it is ATP7B that links copper to ceruloplasmin and releases it into the bloodstream, as well as removing excess copper by secreting it into bile. Both functions of ATP7B are impaired in Wilson's disease. Copper accumulates in the liver tissue; ceruloplasmin is still secreted, but in a form that lacks copper (termed apoceruloplasmin) and rapidly degraded in the bloodstream.
When the amount of copper in the liver overwhelms the proteins that normally bind it, it causes oxidative damage through a process known as Fenton chemistry; this damage eventually leads to chronic active hepatitis, fibrosis(deposition of connective tissue) and cirrhosis. The liver also releases copper into the bloodstream that is not bound to ceruloplasmin. This free copper precipitates throughout the body but particularly in the kidneys, eyes and brain. In the brain, most copper is deposited in the basal ganglia, particularly in the putamen and globus pallidus (together called the lenticular nucleus); these areas normally participate in the coordination of movement as well as playing a significant role in neurocognitive processes such as the processing of stimuli and mood regulation. Damage to these areas, again by Fenton chemistry, produces the neuropsychiatric symptoms seen in Wilson's disease.
It is not clear why Wilson's disease causes hemolysis, but various lines of evidence suggest that high levels of free (non-ceruloplasmin bound) copper have a direct effect on either oxidation of hemoglobin, inhibition of energy-supplying enzymes in the red blood cell, or direct damage to the cell membrane.[1]
References
- ↑ Lee, GR (1999). "Chapter 48: acquired hemolytic anaemias resulting from direct effects of infectious, chemical or physical agents". In Lee GR, Foerster J, Lukens J; et al. Wintrobe's clinical hematology. vol 1 (10th ed.). Williams & Wilkins. p. 1298. ISBN 0-683-18242-0.