Vancomycin-resistant Staphylococcus aureus
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Vancomycin-resistant Staphylococcus aureus (VRSA) is a strain of Staphylococcus aureus that has become resistant to the glycopeptide antibiotic vancomycin. With the increase of staphylococcal resistance to methicillin, vancomycin (or teicoplanin) is often a treatment of choice in infections with methicillin-resistant Staphylococcus aureus (MRSA).
Vancomycin resistance is still a rare occurrence. Unfortunately, VRSA may also be resistant to meropenem and imipenem, two other antibiotics that can be used in sensitive staphylococcus strains.
VISA (vancomycin-intermediate Staphylococcus aureus) was first identified in Japan in 1996 and has since been found in hospitals in England, France, the U.S., Asia and Brazil. It is also termed GISA (glycopeptide-intermediate Staphylococcus aureus) indicating resistance to all glycopeptide antibiotics. These bacterial strains present a thickening of the cell wall which is believed to deplete the vancomycin available to kill the bacteria. This worries many physicians and microbiologists because it leads to high level resistance to vancomycin in Staphylococcus aureus. This has the potential of spreading rapidly throughout large populations of Staphylococcus aureus in hospitals, as opposed to the traditional VISA mode of intermediate resistance to vancomycin, which has to be acquired by the bacterium during treatment with this drug.
Even with the absence of high-level resistance to vancomycin, another concern posed by the presence of VISA is the increased difficulty in prescribing treatments, especially in situations where an effective treatment for an infection is needed urgently, before detailed resistance profiles can be obtained. In hospitals already endemic with multiresistant MRSA, the appearance of VRSA would make the treatment of infected patients much more difficult.
At present, high-level resistances to both glycopeptide and β-lactam antibiotics in Staphylococcus aureus seem to be mutually exclusive, in that both resistances are not seen at once in the same strain of bacterium. However, this is not due to a fundamental biochemical incompatibility. Theoretically, a superbug displaying high-level resistances to both classes of antibiotics could evolve given the current selective environment.
VISA and VRSA are specific types of antimicrobial-resistant staph bacteria. While most staph bacteria are susceptible to the antimicrobial agent vancomycin some have developed resistance.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa.html
Epidemiology and Demographics
Staph bacteria are classified as VISA or VRSA based on laboratory tests. Laboratories perform tests to determine if staph bacteria are resistant to antimicrobial agents that might be used for treatment of infections. For vancomycin and other antimicrobial agents, laboratories determine how much of the agent it requires to inhibit the growth of the organism in a test tube. The result of the test is usually expressed as a minimum inhibitory concentration (MIC) or the minimum amount of antimicrobial agent that inhibits bacterial growth in the test tube. Therefore, staph bacteria are classified as VISA if the MIC for vancomycin is 4-8µg/ml, and classified as VRSA if the vancomycin MIC is >16µg/ml.
VISA and VRSA infections are rare. Only sixteen cases of infection caused by VISA (Michigan 1997, New Jersey 1997, New York 1998, Illinois 1999, Minnesota 2000, Nevada 2000, Maryland 2000, and Ohio 2001) and six cases of infection caused by VRSA (Michigan 2002 , Pennsylvania 2002, New York 2004, and 3 from Michigan in 2005) have been reported in the United States.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
Risk Factors
Persons that developed VISA and VRSA infections had several underlying health conditions (such as diabetes and kidney disease), previous infections with methicillin-resistant Staphylococcus aureus (MRSA), tubes going into their bodies (such as intravenous [IV] catheters), recent hospitalizations, and recent exposure to vancomycin and other antimicrobial agents.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
Screening
Laboratories that use automated methods that are not validated for VRSA detection should also include a vancomycin screen agar plate for enhanced detection of VRSA. If possible, laboratories should incorporate the vancomycin agar screen plate for testing all S. aureus. Alternatively, the screening may be limited to MRSA isolates, since nearly all VISA and all VRSA reported to date (i.e., April 2006) were also MRSA. Laboratories using disk diffusion to determine vancomycin susceptibility should consider adding a second method for VISA detection. The vancomycin screen plate is useful for detecting VISA (MIC = 8 µg/ml). Reliable detection of VISA (MIC = 4 µg/ml) may require a non-automated MIC method.
What is the vancomycin agar screen test?
The vancomycin agar screen test uses commercially prepared agar plates to screen pure cultures of bacteria for vancomycin. These plates contain BHI agar and 6 µg/ml of vancomycin. A 10µl inoculum of a 0.5 McFarland suspension should be spotted on the agar using a micropipette (final concentration=106 CFU/ml). Alternatively, a swab may be dipped in the McFarland suspension, the excess liquid expressed, and used to inoculate the vancomycin agar screen plate. For quality control, laboratories should use Entercococcus faecalis ATCC 29212 as the susceptible control and E. faecalis ATCC 51299 as the resistant control. Up to eight isolates can be tested per plate; quality control should be performed each day of testing. Growth of more than one colony is considered a positive result. All staphylococci that grow on these plates should be inspected for purity, and the original clinical isolates should be tested using an FDA-cleared MIC method for confirmation. Plates prepared in-house using various lots of media performed inconsistently and were inferior to those obtained commercially (CDC unpublished data). Performance of commercially prepared plates varies by individual manufacturer.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html
Pathophysiology & Etiology
VISA and VRSA are specific types of antimicrobial-resistant staph bacteria.
What is Staphylococcus aureus?
Staphylococcus aureus, often simply referred to simply as “staph”, are bacteria commonly found on the skin and in the noses of healthy people. Occasionally, staph can cause infection; staph bacteria are one of the most common causes of skin infections in the United States.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3 http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#1
Natural History
References
References
Laboratory Findings
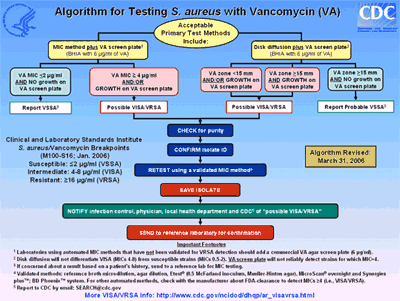
All laboratories should develop a step-by-step problem-solving procedure or algorithm for detecting VISA/VRSA that is specific for their laboratory. A sample algorithm is available here and highlights the recommended testing methodologies for detecting VISA/VRSA and actions based on results.
Not all susceptibility testing methods detect VISA and VRSA isolates. Three out of six confirmed VRSA isolates were not reliably detected by automated testing systems in a recent report. Subsequently, some manufacturers have optimized their systems for VRSA detection, so laboratories should check with manufacturers to determine if their system has FDA clearance for VRSA detection. VRSA are detected by reference broth microdilution, agar dilution, Etest®, MicroScan® overnight and Synergies plus™; BD Phoenix™ system, disk diffusion, and the vancomycin screen agar plate [brain heart infusion (BHI) agar containing 6 µg/ml of vancomycin].
VISA isolates are not detected by disk diffusion. Methods that typically detect VISA are non-automated MIC methods including reference broth microdilution, agar dilution, and Etest® using a 0.5 McFarland standard to prepare inoculum. Automated methods and vancomycin screen agar plates usually detect VISA for which the vancomycin MICs are 8 µg/ml, but further studies are need to define the level of sensitivity of these methods for S. aureus for which the vancomycin MICs are 4 µg/ml.
Electrolyte and Biomarker Studies
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_algo.html http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html
Treatment
VISA and VRSA cannot be successfully treated with vancomycin because these organisms are no longer susceptibile to vancomycin. However, to date, all VISA and VRSA isolates have been susceptible to other Food and Drug Administration (FDA) approved drugs.
Pharmacotherapy
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa.html
Primary Prevention
Use of appropriate infection control practices (such as wearing gloves before and after contact with infectious body substances and adherence to hand hygiene) by healthcare personnel can reduce the spread of VISA and VRSA.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
Secondary Prevention
Because VISA and VRSA are only part of the larger problem of antimicrobial resistance in healthcare settings, CDC has started a Campaign to Prevent Antimicrobial Resistance. The campaign centers around four strategies that clinicians can use to prevent antimicrobial resistance: prevent infections; diagnose and treat infections effectively; use antimicrobials wisely; and prevent transmission. A series of evidence-based steps are described that can reduce the development and spread of resistant organisms such as VISA and VRSA.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
References
Additional Resources
- ^ Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 2003;348:1342-7. PMID 12672861.
See also
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.