Trichophyton mentagrophytes and trichophyton rubrum: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 7: | Line 7: | ||
|indication=type I hypersensitivity (i.e. allergy) to fungus | |indication=type I hypersensitivity (i.e. allergy) to fungus | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=anaphylaxis, | |adverseReactions=[[anaphylaxis]], [[fainting]], [[pallor]], [[bradycardia]], [[hypotension[[angioedema]], [[cough]], [[wheezing]], [[conjunctivitis]], [[rhinitis]] and [[urticaria]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=* This product is intended for use by physicians who are experienced in the administration of allergenic extracts or for use under the guidance of an allergy specialist. Skin tests should be performed after the patient's physical well being and allergic history have been evaluated. Patients should be instructed to recognize adverse reaction symptoms and cautioned to contact the physician if symptoms occur. As with all allergenic extracts, severe systemic reactions may occur, and in certain individuals these reactions may cause death. Patients should be observed for at least 20 minutes after skin tests have been completed. Emergency measures as well as personnel trained in their use should be immediately available in the event of a life threatening reaction. | |blackBoxWarningBody=* This product is intended for use by physicians who are experienced in the administration of allergenic extracts or for use under the guidance of an allergy specialist. Skin tests should be performed after the patient's physical well being and allergic history have been evaluated. Patients should be instructed to recognize adverse reaction symptoms and cautioned to contact the physician if symptoms occur. As with all allergenic extracts, severe systemic reactions may occur, and in certain individuals these reactions may cause death. Patients should be observed for at least 20 minutes after skin tests have been completed. Emergency measures as well as personnel trained in their use should be immediately available in the event of a life threatening reaction. | ||
| Line 18: | Line 18: | ||
* '''INTRADERMAL TEST''' The intradermal test is performed by administering 0.1 mL of extract into the skin. The injection should be given as superficially as possible creating a distinct bleb approximately 5 mm in diameter. The tests should be examined after 15 - 20 minutes and read as negative or positive. Test sites that show a wheal and flare response should be measured and reported in mm of edema and erythema or scored as 1+ to 4+ based on a mm reference scale. Induration after 24 - 48 hours can occur in some individuals and also may be recorded in mm. | * '''INTRADERMAL TEST''' The intradermal test is performed by administering 0.1 mL of extract into the skin. The injection should be given as superficially as possible creating a distinct bleb approximately 5 mm in diameter. The tests should be examined after 15 - 20 minutes and read as negative or positive. Test sites that show a wheal and flare response should be measured and reported in mm of edema and erythema or scored as 1+ to 4+ based on a mm reference scale. Induration after 24 - 48 hours can occur in some individuals and also may be recorded in mm. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |fdaLIADPed=* There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|contraindications=* Conditions under which the administration of allergenic extract may be contraindicated, depending upon individual circumstances, include: EXTREME SENSITIVITY TO AN ALLERGEN - Determined from the allergic history, or from previous anaphylaxis following skin testing or subcutaneous injection; [[MYOCARDIAL INFARCTION]] - Patients who have experienced a recent [[myocardial infarction]] may be less able to tolerate the life-threatening effects of a serious adverse reaction. ASTHMA - Patients with highly unstable asthma are at greater risk of fatal reactions from skin tests than are patients without asthma, especially during seasonal exacerbations of the disease. Also, the combination of unstable [[asthma]] and treatment with [[B-adrenergic blockers]] appears to increase this risk5. Allergenic extract should be temporarily withheld from patients if any of the following conditions exist: 1) severe symptoms of [[hay fever]] and/or [[asthma]]; 2) infection or flu accompanied by [[fever]]; and 3) exposure to excessive amounts of clinically relevant allergen(s) prior to skin testing. | |contraindications=* Conditions under which the administration of allergenic extract may be contraindicated, depending upon individual circumstances, include: EXTREME SENSITIVITY TO AN ALLERGEN - Determined from the allergic history, or from previous anaphylaxis following skin testing or subcutaneous injection; [[MYOCARDIAL INFARCTION]] - Patients who have experienced a recent [[myocardial infarction]] may be less able to tolerate the life-threatening effects of a serious adverse reaction. ASTHMA - Patients with highly unstable asthma are at greater risk of fatal reactions from skin tests than are patients without asthma, especially during seasonal exacerbations of the disease. Also, the combination of unstable [[asthma]] and treatment with [[B-adrenergic blockers]] appears to increase this risk5. Allergenic extract should be temporarily withheld from patients if any of the following conditions exist: 1) severe symptoms of [[hay fever]] and/or [[asthma]]; 2) infection or flu accompanied by [[fever]]; and 3) exposure to excessive amounts of clinically relevant allergen(s) prior to skin testing. | ||
|warnings=* Physicians who use allergenic extracts should have a knowledge and practical understanding of allergy skin testing as described in the published literature6,7,8. | |warnings=* Physicians who use allergenic extracts should have a knowledge and practical understanding of allergy skin testing as described in the published literature6,7,8. | ||
| Line 52: | Line 52: | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | * There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=* There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when allergenic extract is administered to a nursing woman. | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when allergenic extract is administered to a nursing woman. | ||
|useInPed=* The procedures and precautions that are observed in skin testing adults should be observed with children13. | |useInPed=* The procedures and precautions that are observed in skin testing adults should be observed with children13. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=* There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=* There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=* There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |useInRenalImpair=* There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |useInHepaticImpair=* There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=* There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=* There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Intravenous | |administration=* Intravenous | ||
* Prior to administering skin tests, the skin should be cleaned with alcohol and allowed to dry completely. The tests should be placed on the volar surface of the forearm and four rows may be placed on the back. | * Prior to administering skin tests, the skin should be cleaned with alcohol and allowed to dry completely. The tests should be placed on the volar surface of the forearm and four rows may be placed on the back. | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=* There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
|IVCompat=* There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|overdose=* The signs and symptoms of overdosage are the same as those listed under adverse reactions. | |overdose=* The signs and symptoms of overdosage are the same as those listed under adverse reactions. | ||
* The treatment of a systemic allergic reaction resulting from skin tests should include the following: | * The treatment of a systemic allergic reaction resulting from skin tests should include the following: | ||
| Line 82: | Line 82: | ||
* Maintenance of an open airway is critical if upper airway obstruction is present. Corticosteroids may provide benefit if symptoms are prolonged or recurrent. | * Maintenance of an open airway is critical if upper airway obstruction is present. Corticosteroids may provide benefit if symptoms are prolonged or recurrent. | ||
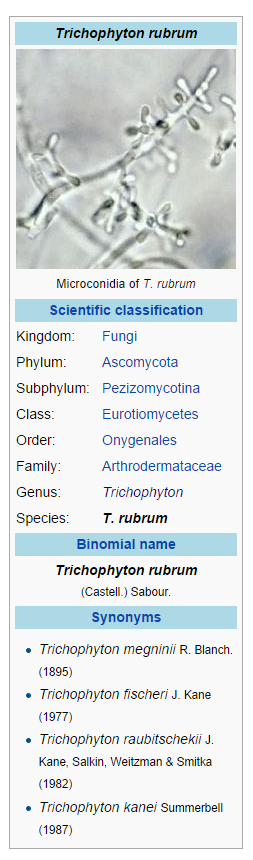
| | |drugBox=[[File:Trichophyton rubrum.png |thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
|structure=* Trichophyton extract for diagnostic skin testing is a sterile solution that is prepared by extracting allergenic source material of equal parts of Trichophyton mentagrophytes and Trichophyton rubrum with an aqueous solution of 0.25% sodium chloride, 0.125% sodium bicarbonate and 50% glycerol v/v. Phenol is added at 0.4% w/v as a preservative. Extract for intradermal administration is diluted with the above solution without glycerol. | |structure=* Trichophyton extract for diagnostic skin testing is a sterile solution that is prepared by extracting allergenic source material of equal parts of Trichophyton mentagrophytes and Trichophyton rubrum with an aqueous solution of 0.25% sodium chloride, 0.125% sodium bicarbonate and 50% glycerol v/v. Phenol is added at 0.4% w/v as a preservative. Extract for intradermal administration is diluted with the above solution without glycerol. | ||
* WEIGHT BY VOLUME (W/V) Weight by volume refers to the weight of raw product added to a measured volume of extraction solution. A 1:500 w/v extract contains 1 gram of source material in 500 mL of solution. The w/v designation refers to concentration rather than potency. Extract labeled w/v has no U.S. Standard of Potency. | * WEIGHT BY VOLUME (W/V) Weight by volume refers to the weight of raw product added to a measured volume of extraction solution. A 1:500 w/v extract contains 1 gram of source material in 500 mL of solution. The w/v designation refers to concentration rather than potency. Extract labeled w/v has no U.S. Standard of Potency. | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |PK=* There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | ||
|nonClinToxic=CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY | |nonClinToxic=CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY | ||
* Long-term studies in animals have not been conducted with allergenic extracts to determine their potential for carcinogenicity, mutagenicity or impairment of fertility. | * Long-term studies in animals have not been conducted with allergenic extracts to determine their potential for carcinogenicity, mutagenicity or impairment of fertility. | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=* There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
|howSupplied=* One mL of extract for intradermal skin testing is supplied in 2 mL sealed multidose vials. Each vial contains enough extract for 10 tests. | |howSupplied=* One mL of extract for intradermal skin testing is supplied in 2 mL sealed multidose vials. Each vial contains enough extract for 10 tests. | ||
|storage=* Extract should be stored at 2°C to 8°C, since higher temperatures may adversely affect the stability of allergens. Do not freeze. | |storage=* Extract should be stored at 2°C to 8°C, since higher temperatures may adversely affect the stability of allergens. Do not freeze. | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=* There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* TRICHOPHYTON FOR INTRADERMAL SKIN TESTING ®<ref>{{Cite web | title =TRICHOPHYTON FOR INTRADERMAL SKIN TESTING- trichophyton mentagrophytes and trichophyton rubrum injection | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aaeffe62-b538-43ca-a3b9-47e28b765d89 }}</ref> | |brandNames=* TRICHOPHYTON FOR INTRADERMAL SKIN TESTING ®<ref>{{Cite web | title =TRICHOPHYTON FOR INTRADERMAL SKIN TESTING- trichophyton mentagrophytes and trichophyton rubrum injection | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aaeffe62-b538-43ca-a3b9-47e28b765d89 }}</ref> | ||
Revision as of 12:51, 30 March 2015
{{DrugProjectFormSinglePage |authorTag=Kiran Singh, M.D. [1] |genericName=trichophyton mentagrophytes and trichophyton rubrum |aOrAn=an |drugClass=antifungal |indicationType=diagnosis |indication=type I hypersensitivity (i.e. allergy) to fungus |hasBlackBoxWarning=Yes |adverseReactions=anaphylaxis, fainting, pallor, bradycardia, [[hypotensionangioedema, cough, wheezing, conjunctivitis, rhinitis and urticaria |blackBoxWarningTitle=ConditionName: |blackBoxWarningBody=* This product is intended for use by physicians who are experienced in the administration of allergenic extracts or for use under the guidance of an allergy specialist. Skin tests should be performed after the patient's physical well being and allergic history have been evaluated. Patients should be instructed to recognize adverse reaction symptoms and cautioned to contact the physician if symptoms occur. As with all allergenic extracts, severe systemic reactions may occur, and in certain individuals these reactions may cause death. Patients should be observed for at least 20 minutes after skin tests have been completed. Emergency measures as well as personnel trained in their use should be immediately available in the event of a life threatening reaction.
- This product should never be injected intravenously.
|fdaLIADAdult===Indications==
- Intradermal skin tests with Trichophyton extract are indicated for use in persons who are suspected of having Type I hypersensitivity (i.e. allergy) to the fungus.
Dosage
- INTRADERMAL TEST The intradermal test is performed by administering 0.1 mL of extract into the skin. The injection should be given as superficially as possible creating a distinct bleb approximately 5 mm in diameter. The tests should be examined after 15 - 20 minutes and read as negative or positive. Test sites that show a wheal and flare response should be measured and reported in mm of edema and erythema or scored as 1+ to 4+ based on a mm reference scale. Induration after 24 - 48 hours can occur in some individuals and also may be recorded in mm.
|offLabelAdultGuideSupport=* There is limited information regarding Off-Label Guideline-Supported Use of Trichophyton mentagrophytes and trichophyton rubrum in adult patients. |offLabelAdultNoGuideSupport=* There is limited information regarding Off-Label Non–Guideline-Supported Use of Trichophyton mentagrophytes and trichophyton rubrum in adult patients.
|fdaLIADPed=* There is limited information regarding FDA-Labeled Use of Trichophyton mentagrophytes and trichophyton rubrum in pediatric patients.
|offLabelPedGuideSupport=* There is limited information regarding Off-Label Guideline-Supported Use of Trichophyton mentagrophytes and trichophyton rubrum in pediatric patients.
|offLabelPedNoGuideSupport=* There is limited information regarding Off-Label Non–Guideline-Supported Use of Trichophyton mentagrophytes and trichophyton rubrum in pediatric patients.
|contraindications=* Conditions under which the administration of allergenic extract may be contraindicated, depending upon individual circumstances, include: EXTREME SENSITIVITY TO AN ALLERGEN - Determined from the allergic history, or from previous anaphylaxis following skin testing or subcutaneous injection; MYOCARDIAL INFARCTION - Patients who have experienced a recent myocardial infarction may be less able to tolerate the life-threatening effects of a serious adverse reaction. ASTHMA - Patients with highly unstable asthma are at greater risk of fatal reactions from skin tests than are patients without asthma, especially during seasonal exacerbations of the disease. Also, the combination of unstable asthma and treatment with B-adrenergic blockers appears to increase this risk5. Allergenic extract should be temporarily withheld from patients if any of the following conditions exist: 1) severe symptoms of hay fever and/or asthma; 2) infection or flu accompanied by fever; and 3) exposure to excessive amounts of clinically relevant allergen(s) prior to skin testing.
|warnings=* Physicians who use allergenic extracts should have a knowledge and practical understanding of allergy skin testing as described in the published literature6,7,8.
Allergenic extracts are manufactured to assure high potency and therefore have the ability to cause serious local and systemic reactions, including death in highly sensitive patients5. Patients should be informed of the risks of skin testing and instructed in the recognition of symptoms of an adverse allergic reaction.
- Excessively large local reactions or systemic reactions are more likely to occur if the patient is skin tested shortly after exposure to allergens to which he or she is sensitive.
|clinicalTrials=* LOCAL REACTIONS Large, persistent local reactions or minor exacerbations of the patient's allergic symptoms may be treated by local cold applications and/or the use of oral anti-histamines.
- SYSTEMIC REACTIONS Allergenic extracts are highly potent and in highly sensitive individuals can cause systemic symptoms, including anaphylaxis. It cannot be overemphasized that anaphylactic shock is always a possibility under certain unpredictable combinations of circumstances. Other possible systemic reaction symptoms may include fainting, pallor, bradycardia, hypotension, angioedema, cough, wheezing, conjunctivitis, rhinitis and urticaria. Therefore, it is imperative that physicians administering skin tests understand and be prepared to treat severe allergic reactions.
- If a systemic or anaphylactic reaction occurs during or following skin testing, the patient should be treated with 1:1000 epinephrine hydrochloride. The recommended dose: infants to 2 years of age 0.05 to 0.1 mL, children 2 to 6 years, 0.15 mL, children 6 to 12 years, 0.2 mL, adults 0.3 - 0.5 mL. If necessary, treatment may be repeated up to three times every 10 - 15 minutes.
- After administration of epinephrine, profound shock or vasomotor collapse should be treated with intravenous fluids and possibly vasoactive drugs. Oxygen should be given by mask. Intravenous antihistamine, aminophylline, inhaled bronchodilators or adrenal corticosteroids may be used if necessary after adequate epinephrine and circulatory support have been given. Emergency resuscitation measures and personnel trained in their use should be available immediately. The physician should be prepared in advance for all contingencies. Promptness in beginning emergency treatment is of utmost importance14.
- Serious adverse reactions should be reported to MedWatch: The FDA Safety Information and Adverse Event Reporting Program, Office Of The Center Director, Center for Drug Evaluation and Research, 5515 Security Lane, Suite 5100, Rockville, MD 20852, Telephone (800) 332-1088.
|postmarketing=There is limited information regarding Postmarketing Experience of Trichophyton mentagrophytes and trichophyton rubrum in the drug label. |drugInteractions=* Treatment with beta-blocking drugs may make patients refractory to the usual dose of epinephrine, in the event epinephrine is required to control an adverse reaction11.
- Since drugs may affect the reactivity of the skin, patients should be instructed to avoid medications, particularly antihistamines and sympathomimetic drugs for at least 24 hours prior to skin testing. Non-sedating antihistamine suppresses the erythema/edema response for longer periods and should be withheld according to information included in the package insert. Adrenal corticosteroids and ACTH do not alter the immediate hypersensitivity reaction of the skin but do reduce delayed-type reactions associated with cellular hypersensitivity.
|useInPregnancyFDA=PREGNANCY CATEGORY C Allergenic Extracts. Animal reproduction studies have not been conducted with allergenic extracts. It is also not known whether allergenic extracts can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Allergenic extracts should be given to a pregnant woman only if clearly needed. Since studies indicate no increased risk to the fetus or to the mother who is treated cautiously with immunotherapy during a normal pregnancy, it is unlikely that extract administered by skin test will be harmful. However, on the basis of histamine's known ability to contract uterine muscle, extensive testing with its possibility of histamine release should be avoided during pregnancy. Also, immunologic suppressive action can occur during pregnancy and for this reason it is possible that the results of allergy skin tests may not accurately reflect the allergic state of the pregnant patient12. |useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trichophyton mentagrophytes and trichophyton rubrum in women who are pregnant.
|useInLaborDelivery=* There is no FDA guidance on use of Trichophyton mentagrophytes and trichophyton rubrum during labor and delivery. |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when allergenic extract is administered to a nursing woman. |useInPed=* The procedures and precautions that are observed in skin testing adults should be observed with children13. |useInGeri=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum with respect to geriatric patients. |useInGender=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum with respect to specific gender populations. |useInRace=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum with respect to specific racial populations. |useInRenalImpair=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum in patients with renal impairment. |useInHepaticImpair=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum in patients with hepatic impairment. |useInReproPotential=* There is no FDA guidance on the use of Trichophyton mentagrophytes and trichophyton rubrum in women of reproductive potentials and males. |useInImmunocomp=* There is no FDA guidance one the use of Trichophyton mentagrophytes and trichophyton rubrum in patients who are immunocompromised. |administration=* Intravenous
- Prior to administering skin tests, the skin should be cleaned with alcohol and allowed to dry completely. The tests should be placed on the volar surface of the forearm and four rows may be placed on the back.
|monitoring=* There is limited information regarding Monitoring of Trichophyton mentagrophytes and trichophyton rubrum in the drug label.
|IVCompat=* There is limited information regarding IV Compatibility of Trichophyton mentagrophytes and trichophyton rubrum in the drug label.
|overdose=* The signs and symptoms of overdosage are the same as those listed under adverse reactions.
- The treatment of a systemic allergic reaction resulting from skin tests should include the following:
- The patient should be placed in the recumbent position to maintain blood flow to the head.
- Aqueous epinephrine 1:1,000 should be administered subcutaneously.
- Reassurance should be provided.
- The above steps should be performed nearly simultaneously and as soon as possible after the reaction begins. Persistent wheezing may necessitate treatment with intravenous aminophylline and inhaled bronchodilators. For profound shock and hypotension, intravenous fluids, vasopressors and oxygen also may be needed.
- Maintenance of an open airway is critical if upper airway obstruction is present. Corticosteroids may provide benefit if symptoms are prolonged or recurrent.
|drugBox=
|structure=* Trichophyton extract for diagnostic skin testing is a sterile solution that is prepared by extracting allergenic source material of equal parts of Trichophyton mentagrophytes and Trichophyton rubrum with an aqueous solution of 0.25% sodium chloride, 0.125% sodium bicarbonate and 50% glycerol v/v. Phenol is added at 0.4% w/v as a preservative. Extract for intradermal administration is diluted with the above solution without glycerol.
- WEIGHT BY VOLUME (W/V) Weight by volume refers to the weight of raw product added to a measured volume of extraction solution. A 1:500 w/v extract contains 1 gram of source material in 500 mL of solution. The w/v designation refers to concentration rather than potency. Extract labeled w/v has no U.S. Standard of Potency.
|PD=There is limited information regarding Pharmacodynamics of Trichophyton mentagrophytes and trichophyton rubrum in the drug label. |PK=* There is limited information regarding Pharmacokinetics of Trichophyton mentagrophytes and trichophyton rubrum in the drug label. |nonClinToxic=CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- Long-term studies in animals have not been conducted with allergenic extracts to determine their potential for carcinogenicity, mutagenicity or impairment of fertility.
|clinicalStudies=* There is limited information regarding Clinical Studies of Trichophyton mentagrophytes and trichophyton rubrum in the drug label. |howSupplied=* One mL of extract for intradermal skin testing is supplied in 2 mL sealed multidose vials. Each vial contains enough extract for 10 tests. |storage=* Extract should be stored at 2°C to 8°C, since higher temperatures may adversely affect the stability of allergens. Do not freeze. |fdaPatientInfo=* There is limited information regarding Patient Counseling Information of Trichophyton mentagrophytes and trichophyton rubrum in the drug label. |alcohol=* Alcohol-Trichophyton mentagrophytes and trichophyton rubrum interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=* TRICHOPHYTON FOR INTRADERMAL SKIN TESTING ®[1] |lookAlike=* A® — B®[2] |drugShortage= }} {{#subobject:
|Page Name=Trichophyton mentagrophytes and trichophyton rubrum
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Trichophyton mentagrophytes and trichophyton rubrum |Label Name=Trichophyton mentagrophytes and trichophyton rubrum11.png
}}
{{#subobject:
|Label Page=Trichophyton mentagrophytes and trichophyton rubrum |Label Name=Trichophyton mentagrophytes and trichophyton rubrum11.png
}}