Triamterene
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings
See full prescribing information for complete Boxed Warning.
* Abnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-sparing agents, including Dyrenium. Hyperkalemia is more likely to occur in patients with renal impairment and diabetes (even without evidence of renal impairment), and in the elderly or severely ill. Since uncorrected hyperkalemia may be fatal, serum potassium levels must be monitored at frequent intervals especially in patients receiving Dyrenium, when dosages are changed or with any illness that may influence renal function.
|
Overview
Triamterene is a potassium-sparing diuretic that is FDA approved for the {{{indicationType}}} of edema associated with congestive heart failure, cirrhosis, nephrotic syndrome, steroid, and secondary hyperaldosteronism. There is a Black Box Warning for this drug as shown here. Common adverse reactions include electrolyte disturbances and hyperuricemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Edema
- Dyrenium (triamterene) is indicated in the treatment of edema associated with congestive heart failure, cirrhosis of the liver and the nephrotic syndrome; steroid-induced edema, idiopathic edema and edema due to secondary hyperaldosteronism.
- Dyrenium may be used alone or with other diuretics, either for its added diuretic effect or its potassium-sparing potential. It also promotes increased diuresis when patients prove resistant or only partially responsive to thiazides or other diuretics because of secondary hyperaldosteronism.
- When Dyrenium (triamterene) is added to other diuretic therapy or when patients are switched to Dyrenium from other diuretics, all potassium supplementation should be discontinued.
- Dosing Information
- When used alone, the usual starting dose is 100 mg PO bid after meals.
- When combined with another diuretic or antihypertensive agent, the total daily dosage of each agent should usually be lowered initially and then adjusted to the patient’s needs. The total daily dosage should not exceed 300 mg.
Usage in Pregnancy
- The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
- Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Diuretics are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy. Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Triamterene in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Triamterene in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Triamterene in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Triamterene in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Triamterene in pediatric patients.
Contraindications
- Condition1
Warnings
|
Warnings
See full prescribing information for complete Boxed Warning.
* Abnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-sparing agents, including Dyrenium. Hyperkalemia is more likely to occur in patients with renal impairment and diabetes (even without evidence of renal impairment), and in the elderly or severely ill. Since uncorrected hyperkalemia may be fatal, serum potassium levels must be monitored at frequent intervals especially in patients receiving Dyrenium, when dosages are changed or with any illness that may influence renal function.
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Triamterene in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Triamterene in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Triamterene in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Triamterene during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Triamterene with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Triamterene with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Triamterene with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Triamterene with respect to specific gender populations.
Race
There is no FDA guidance on the use of Triamterene with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Triamterene in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Triamterene in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Triamterene in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Triamterene in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Triamterene in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Triamterene in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Triamterene in the drug label.
Pharmacology
There is limited information regarding Triamterene Pharmacology in the drug label.
Mechanism of Action
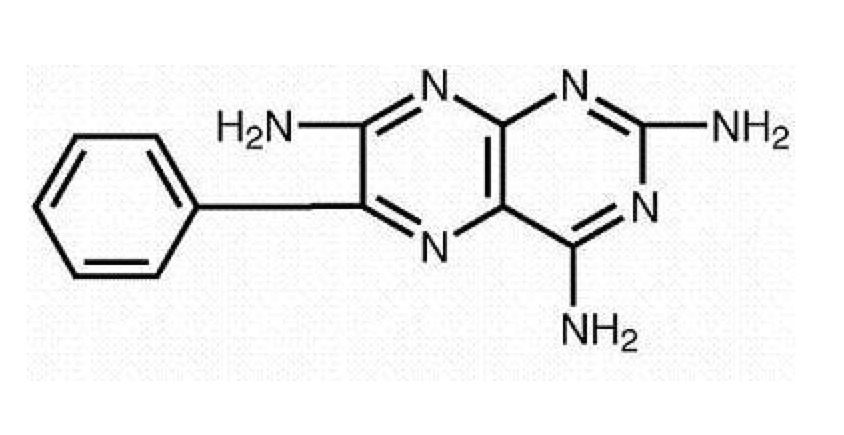
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Triamterene in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Triamterene in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Triamterene in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Triamterene in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Triamterene Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Triamterene |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Triamterene |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Triamterene in the drug label.
Precautions with Alcohol
- Alcohol-Triamterene interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dyrenium®[1]
Look-Alike Drug Names
- N/A[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "DYRENIUM (triamterene) capsule".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Triamterene |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Triamterene |Label Name=Triamterene11.png
}}
{{#subobject:
|Label Page=Triamterene |Label Name=Triamterene11.png
}}