Toremifene: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| (14 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{RB}} | ||
|genericName=Toremifene Citrate | |genericName=Toremifene Citrate | ||
|aOrAn= | |aOrAn=an | ||
|drugClass=antineoplasic agent | |drugClass=[[Anti-estrogen therapy|antiestrogen]] antineoplasic agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors | |indication=[[breast cancer|metastatic breast cancer]] in [[postmenopausal women]] with [[Estrogen receptor|estrogen-receptor positive]] or unknown tumors | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=hot flashes, sweating, nausea and vaginal discharge | |adverseReactions=[[hot flashes]], [[sweating]], [[nausea]] and [[vaginal discharge]] | ||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
| Line 15: | Line 13: | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">WARNING: QT PROLONGATION : </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">WARNING: QT PROLONGATION : </span></i> | ||
* | * Toremifene has been shown to prolong the QTc interval in a dose-and-concentration-related manner. Prolongation of the QT interval can result in a type of ventricular tachycardia called Torsade de pointes, which may result in syncope, seizure, and/or death. Toremifene should not be prescribed to patients with congenital/acquired QT prolongation, uncorrected hypokalemia or uncorrected hypomagnesemia. Drugs known to prolong the QT interval and strong CYP3A4 inhibitors should be avoided | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
| Line 21: | Line 19: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=====Indications==== | |fdaLIADAdult=====Indications==== | ||
* | * Toremifene® is an [[estrogen agonist]]/[[estrogen antagonist|antagonist]] indicated for the treatment of [[metastatic breast cancer]] in [[postmenopausal women]] with [[Estrogen receptor|estrogen-receptor positive]] or unknown tumors. | ||
====Dosage==== | ====Dosage==== | ||
* The dosage of | * The dosage of Toremifene is 60 mg, once daily, orally. Treatment is generally continued until disease progression is observed. | ||
====DOSAGE FORMS AND STRENGTHS==== | ====DOSAGE FORMS AND STRENGTHS==== | ||
* Tablet is 60 mg, round, convex, unscored, uncoated, and white, or almost white, identified with TO 60 embossed on one side. | * Tablet is 60 mg, round, convex, unscored, uncoated, and white, or almost white, identified with TO 60 embossed on one side. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Toremifene Citrate in adult patients. | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
| Line 36: | Line 34: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of Toremifene Citrate in pediatric patients. | ||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Toremifene Citrate in pediatric patients. | ||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Toremifene Citrate in pediatric patients. | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications======Hypersensitivity to the Drug===== | ||
* Toremifene is contraindicated in patients with known [[hypersensitivity]] to the drug. | |||
=====QT Prolongation, Hypokalemia, Hypomagnesemia===== | |||
* Toremifene should not be prescribed to patients with [[congenital/acquired QT prolongation]] ([[long QT syndrome]]), uncorrected [[hypokalemia]], or uncorrected [[hypomagnesemia]]. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* | |warnings======Prolongation of the QT Interval===== | ||
* Toremifene has been shown to [[prolong the QTc interval]] in a dose- and concentration-related manner. Prolongation of the [[QT interval]] can result in a type of [[ventricular tachycardia]] called [[Torsade de pointes]], which may result in [[syncope]], [[seizure]], and/or death. | |||
* Toremifene should be avoided in patients with [[long QT syndrome]]. Caution should be exercised in patients with [[congestive heart failure]], [[hepatic impairment]] and [[electrolyte abnormalities]]. [[Hypokalemia]] or [[hypomagnesemia]] must be corrected prior to initiating toremifene and these electrolytes should be monitored periodically during therapy. Drugs that prolong the [[QT interval]] should be avoided. In patients at increased risk, [[electrocardiograms]] (ECGs) should be obtained at baseline and as clinically indicated. | |||
* | =====Hypercalcemia and Tumor Flare===== | ||
* As with other [[antiestrogens]], [[hypercalcemia]] and tumor flare have been reported in some [[breast cancer]] patients with bone metastases during the first weeks of treatment with Toremifene. Tumor flare is a syndrome of diffuse musculoskeletal pain and [[erythema]] with increased size of tumor lesions that later regress. It is often accompanied by [[hypercalcemia]]. [[Tumor flare]] does not imply failure of treatment or represent tumor progression. If [[hypercalcemia]] occurs, appropriate measures should be instituted and, if [[hypercalcemia]] is severe, Toremifene treatment should be discontinued. | |||
=====Tumorigenicity===== | |||
* Since most toremifene trials have been conducted in patients with metastatic disease, adequate data on the potential endometrial tumorigenicity of long-term treatment with Toremifene are not available. [[Endometrial hyperplasia]] has been reported. Some patients treated with Toremifene have developed [[endometrial cancer]], but circumstances (short duration of treatment or prior antiestrogen treatment or premalignant conditions) make it difficult to establish the role of Toremifene. [[Endometrial hyperplasia]] of the uterus was observed in animals treated with toremifene. | |||
=====General===== | |||
* Patients with a history of thromboembolic diseases should generally not be treated with Toremifene. In general, patients with preexisting [[endometrial hyperplasia]] should not be given long-term Toremifene treatment. Patients with bone metastases should be monitored closely for hypercalcemia during the first weeks of treatment . [[Leukopenia]] and [[thrombocytopenia]] have been reported rarely; leukocyte and platelet counts should be monitored when using Toremifene in patients with [[leukopenia]] and [[thrombocytopenia]]. | |||
===== | =====Laboratory Tests===== | ||
* Periodic [[complete blood counts]], calcium levels, and [[liver function tests]] should be obtained. | |||
=====Use in Pregnancy===== | |||
* Based on its mechanism of action in humans and findings of increased pregnancy loss and fetal malformation in animal studies, Toremifene can cause fetal harm when administered to a pregnant woman. Toremifene caused embryo-fetal toxicities at maternal doses that were lower than the 60 mg daily recommended human dose on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women using Toremifene. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. | |||
=====Women of Childbearing Potential===== | |||
* Toremifene is indicated only in postmenopausal women. However, premenopausal women prescribed Toremifene should use effective non-hormonal contraception and should be apprised of the potential hazard to the fetus should pregnancy occur. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
===== | =====Clinical Trials Experience===== | ||
* Adverse drug reactions are principally due to the [[antiestrogenic]] actions of Toremifene and typically occur at the beginning of treatment. | |||
* The incidences of the following eight clinical toxicities were prospectively assessed in the North American Study. The incidence reflects the toxicities that were considered by the investigator to be drug related or possibly drug related. | |||
: [[File:Toremifene Adv Eff 1.png|none|500px]] | |||
* Approximately 1% of patients receiving Toremifene (n = 592) in the three controlled studies discontinued treatment as a result of adverse reactions ([[nausea and vomiting]], [[fatigue]], [[thrombophlebitis]], [[depression]], [[lethargy]], [[anorexia]], [[ischemic attack]], [[arthritis]], [[pulmonary embolism]], and [[myocardial infarction]]). | |||
* Serious adverse reactions occurring in at least 1% of patients receiving Toremifene in the three major trials are listed in the table below. | |||
* Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted. The patients were randomized to parallel groups receiving Toremifene 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively | |||
: [[File:Toremifene Adv Eff 2.png|none|500px]] | |||
* Other adverse reactions included [[leukopenia]] and [[thrombocytopenia]], [[skin discoloration]] or [[dermatitis]], [[constipation]], [[dyspnea]], [[paresis]], [[tremor]], [[vertigo]], [[pruritu]]s, [[anorexia]], reversible [[corneal opacity]] (corneal verticulata), [[asthenia]], [[alopecia]], [[depression]], [[jaundice]], and rigors. | |||
* The incidence of [[AST]] elevations was greater in the 200 and 240 mg Toremifene dose arms than in the tamoxifen arms. Higher doses of Toremifene were also associated with an increase in [[nausea]]. | |||
* Approximately 4% of patients were withdrawn for toxicity from the high-dose Toremifene treatment arms. Reasons for withdrawal included [[hypercalcemia]], abnormal liver function tests, and one case each of toxic [[hepatitis]], [[depression]], [[dizziness]], incoordination, [[ataxia]], [[blurry vision]], [[diffuse dermatitis]], and a constellation of symptoms consisting of [[nausea]], [[sweating]], and [[tremor]]. | |||
|postmarketing=* The following adverse reactions were identified during post approval use of Toremifene. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
* Adverse reactions reported during post approval use of Toremifene have been consistent with clinical trial experience. The most frequently reported adverse reactions related to Toremifene use since market introduction include [[hot flash]], [[sweating]], [[nausea]], and [[vaginal discharge]]. | |||
|drugInteractions======Drugs that Decrease Renal Calcium Excretion===== | |||
* Drugs that decrease renal calcium excretion, e.g., [[thiazide diuretic]]s, may increase the risk of [[hypercalcemia]] in patients receiving Toremifene. | |||
=====Agents that Prolong QT===== | |||
* The administration of Toremifene with agents that have demonstrated [[QT prolongation]] as one of their pharmacodynamic effects should be avoided. Should treatment with any of these agents be required, it is recommended that therapy with Toremifene be interrupted. If interruption of treatment with Toremifene is not possible, patients who require treatment with a drug that prolongs QT should be closely monitored for prolongation of the QT interval. Agents generally accepted to [[prolong QT interval]] include Class 1A (e.g., [[quinidine]], [[procainamide]], [[disopyramide]]) and Class III (e.g., [[amiodarone]], [[sotalol]], [[ibutilide]], dofetilide) [[antiarrhythmics]]; certain [[antipsychotics]] (e.g., [[thioridazine]], [[haloperidol]]); certain antidepressants (e.g., [[venlafaxine]], [[amitriptyline]]); certain antibiotics (e.g., [[erythromycin]], [[clarithromycin]], [[levofloxacin]], [[ofloxacin]]); and certain anti-emetics (e.g., [[ondansetron]], [[granisetron]]). In patients at increased risk, [[electrocardiograms]] (ECGs) should be obtained and patients monitored as clinically indicated. | |||
===== | =====Effect of Strong CYP3A4 Inducers on Toremifene===== | ||
* Strong [[CYP3A4]] enzyme inducers, such as [[dexamethasone]], [[phenytoin]], [[carbamazepine]], [[rifampin]], [[rifabutin]], [[phenobarbital]], St. John's Wort, lower the steady-state concentration of toremifene in serum. | |||
=====Effect of Strong CYP3A4 Inhibitors on Toremifene===== | |||
* In a study of 18 healthy subjects, 80 mg toremifene once daily coadministered with 200 mg of [[ketoconazole]] twice daily increased the toremifene [[Cmax]] and [[AUC]] by 1.4- and 2.9-fold, respectively. N-demethyltoremifene Cmax and AUC were reduced by 56% and 20%, respectively. | |||
* The administration of Toremifene with agents that are strong [[CYP3A4]] inhibitors (e.g., [[ketoconazole]], [[itraconazole]], [[clarithromycin]], [[atazanavir]], [[indinavir]], [[nefazodone]], [[nelfinavir]], [[ritonavir]], [[saquinavir]], [[telithromycin]], and [[voriconazole]]) increase the steady-state concentration in serum and should be avoided. Grapefruit juice may also increase plasma concentrations of toremifene and should be avoided. Should treatment with any of these agents be required, it is recommended that therapy with Toremifene be interrupted. If interruption of treatment with Toremifene is not possible, patients who require treatment with a drug that strongly inhibits CYP3A4 should be closely monitored for prolongation of the [[QT interval]]. | |||
=====Effect of Toremifene on CYP3A4 Substrates===== | |||
* In a study of 20 healthy subjects, 2 mg midazolam once daily (days 6 and 18) coadministered with toremifene as a 480 mg loading dose followed by 80 mg once daily for 16 days. Following coadministration on days 6 and 18 relevant increases in [[midazolam]] and α-hydroxymidazolam Cmax and AUC were not observed. Following coadministration on day 18 midazolam and α-hydroxymidazolam [[Cmax]] and [[AUC]] were reduced by less than 20%. | |||
* Clinically relevant exposure changes in sensitive substrates due to inhibition or induction of [[CYP3A4]] by toremifene appear unlikely. | |||
=====Effect of Toremifene on CYP2C9 Substrates===== | |||
* In a study of 20 healthy subjects, 500 mg tolbutamide once daily (days 7 and 19) coadministered with toremifene as a 480 mg loading dose followed by 80 mg once daily for 16 days. Following coadministration on days 7 and 19 plasma tolbutamide [[Cmax]] and [[AUC]] were increased by less than 30%. A reduction of similar magnitude was observed for [[hydroxytolbutamide]] and [[carboxytolbutamide]] [[Cmax]] and [[AUC]]. | |||
* Toremifene is a weak inhibitor of [[CYP2C9]]. Concomitant use of [[CYP2C9]] substrates with a narrow therapeutic index such as warfarin or phenytoin with Toremifene should be done with caution and requires careful monitoring (e.g., substrate concentrations (if possible), appropriate laboratory markers, and signs and symptoms of increased exposure). | |||
<!--Use in Specific Populations--> | |||
|FDAPregCat=D | |||
|useInPregnancyFDA=* Based on its mechanism of action in humans and findings of increased pregnancy loss and fetal malformation in animal studies, Toremifene can cause fetal harm when administered to a pregnant woman. Toremifene caused embryo-fetal toxicities at maternal doses that were lower than the 60 mg daily recommended human dose on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women using Toremifene. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. | |||
* In animal studies, toremifene crossed the placenta and accumulated in the rodent fetus. Administration of toremifene to pregnant rats during organogenesis at doses of approximately 6% the daily maximum recommended human dose of 60 mg (on a mg/m2 basis) resulted in signs of maternal toxicity and increased preimplantation loss, increased resorptions, reduced fetal weight, and fetal anomalies. Fetal anomalies include malformation of limbs, incomplete [[ossification]], misshapen bones, ribs/spine anomalies, [[hydroureter]], [[hydronephrosis]], testicular displacement, and [[subcutaneous edema]]. Maternal toxicity may have contributed to these adverse embryo-fetal effects. Similar embryo-fetal toxicities occurred in rabbits that received toremifene at doses approximately 40% the daily recommended human dose of 60 mg (on a mg/m2 basis). Findings in rabbits included increased preimplantation loss, increased resorptions, and fetal anomalies, including incomplete ossification and anencephaly. | |||
* Animal doses resulting in embryo-fetal toxicities were ≥1.0 mg/kg/day in rats and ≥1.25 mg/kg/day in rabbits. | |||
* In rodent models of fetal reproductive tract development, toremifene produced inhibition of uterine development in female pups similar to effects seen with [[diethylstilbestrol]] (DES) and [[tamoxifen]]. The clinical relevance of these changes is not known. Neonatal rodent studies have not been conducted to assess the potential for toremifene to cause other DES-like effects in offspring (i.e., vaginal adenosis). [[Vaginal adenosis]] in animals occurred following treatment with other drugs of this class and has been observed in women exposed to diethylstilbestrol in utero. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Toremifene Citrate in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of Toremifene Citrate during labor and delivery. | |||
|useInNursing=* It is not known if toremifene is excreted in human milk. Toremifene is excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Toremifene, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=* There is no indication for use of Toremifene in pediatric patients. | |||
|useInGeri=* The pharmacokinetics of toremifene were studied in 10 healthy young males and 10 elderly females following a single 120 mg dose under fasting conditions. Increases in the elimination half-life (4.2 versus 7.2 days) and the volume of distribution (457 versus 627 L) of toremifene were seen in the elderly females without any change in clearance or AUC. | |||
== | * The median ages in the three controlled studies ranged from 60 to 66 years. No significant age-related differences in Toremifene effectiveness or safety were noted. | ||
|useInGender=There is no FDA guidance on the use of Toremifene Citrate with respect to specific gender populations. | |||
|useInRace=* The pharmacokinetics of toremifene in patients of different races has not been studied. | |||
* Fourteen percent of patients in the North American Study were non-Caucasian. No significant race-related differences in Toremifene effectiveness or safety were noted. | |||
|useInRenalImpair=* The pharmacokinetics of toremifene and N-demethyltoremifene were similar in normals and in patients with impaired kidney function. | |||
|useInHepaticImpair=* The mean elimination half-life of toremifene was increased by less than twofold in 10 patients with hepatic impairment ([[cirrhosis]] or [[fibrosis]]) compared to subjects with normal hepatic function. The pharmacokinetics of N-demethyltoremifene were unchanged in these patients. Ten patients on [[anticonvulsants]] ([[phenobarbital]], [[clonazepam]], [[phenytoin]], and [[carbamazepine]]) showed a twofold increase in clearance and a decrease in the elimination half-life of toremifene. | |||
|useInReproPotential=There is no FDA guidance on the use of Toremifene Citrate in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of Toremifene Citrate in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Oral | |||
|monitoring=* [[Hypokalemia]] or [[hypomagnesemia]] must be corrected prior to initiating toremifene and these electrolytes should be monitored periodically during therapy. | |||
* Patients with bone metastases should be monitored closely for [[hypercalcemia]] during the first weeks of treatment | |||
* [[leukocyte]] and [[platelet]] counts should be monitored when using Toremifene in patients with [[leukopenia]] and [[thrombocytopenia]]. | |||
* Periodic [[complete blood counts]], calcium levels, and [[liver function tests]] should be obtained. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of Toremifene Citrate in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* Lethality was observed in rats following single oral doses that were ≥1000 mg/kg (about 150 times the recommended human dose on a mg/m2 basis) and was associated with [[gastric atony]]/dilatation leading to interference with digestion and adrenal enlargement. | |||
* [[Vertigo]], [[headache]], and [[dizziness]] were observed in healthy volunteer studies at a daily dose of 680 mg for 5 days. The symptoms occurred in two of the five subjects during the third day of the treatment and disappeared within 2 days of discontinuation of the drug. No immediate concomitant changes in any measured clinical chemistry parameters were found. In a study in postmenopausal breast cancer patients, toremifene 400 mg/m2/day caused dose-limiting nausea, vomiting, and dizziness, as well as reversible hallucinations and ataxia in one patient. | |||
* Theoretically, overdose may be manifested as an increase of antiestrogenic effects, such as hot flashes; estrogenic effects, such as vaginal bleeding; or nervous system disorders, such as [[vertigo]], [[dizziness]], [[ataxia]], and [[nausea]]. There is no specific antidote and the treatment is symptomatic. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 470611877 | |||
| IUPAC_name = 2-{4-[(1''Z'')-4-chloro-1,2-diphenyl-but-1-en-1-yl]phenoxy}-''N,N''-dimethylethanamine | |||
| image = Tormifine Wiki Str.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|monograph|Toremifene}} | |||
| MedlinePlus = a608003 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = more than 99.5% | |||
| metabolism = | |||
| elimination_half-life = 5 days | |||
| excretion = | |||
===== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 89778-26-7 | |||
| ATC_prefix = L02 | |||
| ATC_suffix = BA02 | |||
| ATC_supplemental = | |||
| PubChem = 3005573 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00539 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 2275722 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 7NFE54O27T | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08620 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 9635 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1655 | |||
<!--Chemical data--> | |||
| C=26 | H=28 | Cl=1 | N=1 | O=1 | |||
| molecular_weight = 405.959 g/mol | |||
| smiles = ClCCC(/c1ccccc1)=C(/c2ccc(OCCN(C)C)cc2)c3ccccc3 | |||
| InChI = 1/C26H28ClNO/c1-28(2)19-20-29-24-15-13-23(14-16-24)26(22-11-7-4-8-12-22)25(17-18-27)21-9-5-3-6-10-21/h3-16H,17-20H2,1-2H3/b26-25- | |||
| InChIKey = XFCLJVABOIYOMF-QPLCGJKRBL | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C26H28ClNO/c1-28(2)19-20-29-24-15-13-23(14-16-24)26(22-11-7-4-8-12-22)25(17-18-27)21-9-5-3-6-10-21/h3-16H,17-20H2,1-2H3/b26-25- | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = XFCLJVABOIYOMF-QPLCGJKRSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction=* Toremifene is a nonsteroidal triphenylethylene derivative. Toremifene binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities, depending upon the duration of treatment, animal species, gender, target organ, or endpoint selected. In general, however, nonsteroidal triphenylethylene derivatives are predominantly antiestrogenic in rats and humans and estrogenic in mice. In rats, toremifene causes regression of established dimethylbenzanthracene (DMBA)-induced mammary tumors. The antitumor effect of toremifene in breast cancer is believed to be mainly due to its antiestrogenic effects, i.e., its ability to compete with estrogen for binding sites in the cancer, blocking the growth-stimulating effects of estrogen in the tumor. | |||
<!--Structure--> | |||
|structure=* Toremifene (toremifene citrate) Tablets for oral administration each contain 88.5 mg of toremifene citrate, which is equivalent to 60 mg toremifene. | |||
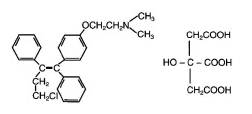
* Toremifene is an estrogen agonist/antagonist. The chemical name of toremifene is: 2-{p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy}-N,N-dimethylethylamine citrate (1:1). The structural formula is: | |||
: [[File:Toremefine Str.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
and the molecular formula is C26H28ClNO • C6H8O7. The molecular weight of toremifene citrate is 598.10. The pKa is 8.0. Water solubility at 37˚C is 0.63 mg/mL and in 0.02N HCl at 37˚C is 0.38 mg/mL. | |||
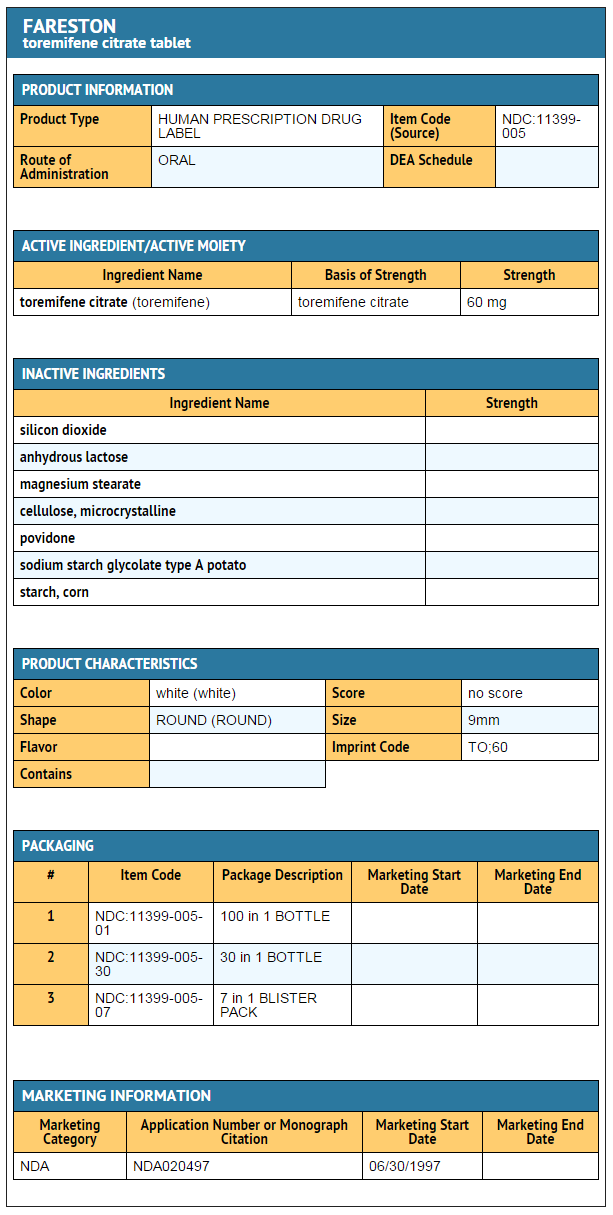
* Toremifene is available only as tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch. | |||
<!--Pharmacodynamics--> | |||
|PD=* Toremifene causes a decrease in the estradiol-induced vaginal cornification index in some postmenopausal women, indicative of its antiestrogenic activity. Toremifene also has estrogenic activity as shown by decreases in serum gonadotropin concentrations (FSH and LH). | |||
===== | =====Effects on Cardiac Electrophysiology===== | ||
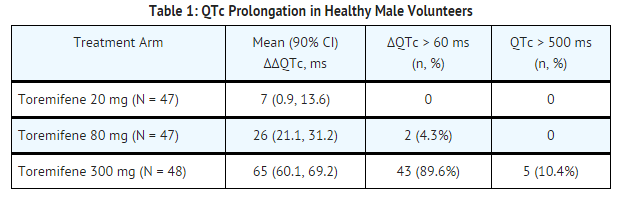
* The effect of 20 mg, 80 mg, and 300 mg of toremifene on [[QT interval]] was evaluated in a double-blind, randomized study in healthy male subjects aged 18 to 45 years. The QT interval was measured at steady state of toremifene (Day 5 of dosing), including the time of peak plasma concentration (Tmax), at 13 time points (4 ECGs/time point) over 24 hours post dose in a time matched analysis. The 300 mg dose of toremifene (approximately five times the highest recommended dose 60 mg) was chosen because this dose produces exposure to toremifene that will cover the expected exposures that may result from potential drug interactions and hepatic impairment. | |||
* Dose and concentration-related increases in the QTc interval and T wave changes were observed (see TABLE 1). These effects are believed to be caused by toremifene and N-demethyltoremifene. Toremifene had no effects on heart rate, PR and QRS interval duration | |||
: [[File:Toremifine PD.png|thumb|none|500px|This image is provided by the National Library of Medicine.]] | |||
===== | <!--Pharmacokinetics--> | ||
|PK======Absorption===== | |||
* Toremifene is well absorbed after oral administration and absorption is not influenced by food. Peak plasma concentrations are obtained within 3 hours. Toremifene displays linear pharmacokinetics after single oral doses of 10 to 680 mg. After multiple dosing, dose proportionality was observed for doses of 10 to 400 mg. Steady state concentrations were reached in about 4-6 weeks. | |||
=====Distribution===== | |||
* Toremifene has an apparent volume of distribution of 580 L and binds extensively (>99.5%) to serum proteins, mainly albumin. | |||
=====Metabolism===== | |||
* Toremifene is extensively metabolized, principally by [[CYP3A4]] to N-demethyltoremifene which is also antiestrogenic but with weak in vivo antitumor potency. Serum concentrations of N-demethyltoremifene are 2 to 4 times higher than toremifene at steady state. | |||
* Following multiple dosing with toremifene in 20 healthy volunteers, plasma toremifene exposure was lower on Day 17 compared to Day 5 by approximately 14%. N-demethyltoremifene exposure was higher on Day 17 compared to Day 5 by approximately 80%. Based on these data and an in vitro induction study in human hepatocytes, auto-induction of CYP3A4 by toremifene is likely. The effect of auto-induction on efficacy was likely captured following prolonged dosing in the clinical studies. | |||
===== | =====Elimination===== | ||
* The plasma concentration time profile of toremifene declines biexponentially after absorption with a mean distribution half-life of about 4 hours and an elimination half-life of about 5 days. Elimination half-lives of major metabolites, N-demethyltoremifene and (Deaminohydroxy) toremifene, were 6 and 4 days, respectively. Mean total clearance of toremifene was approximately 5 L/h. Toremifene is eliminated as metabolites primarily in the feces, with about 10% excreted in the urine during a 1-week period. Elimination of toremifene is slow, in part because of enterohepatic circulation. | |||
=====Renal insufficiency===== | |||
* The pharmacokinetics of toremifene and N-demethyltoremifene were similar in normals and patients with impaired kidney function. | |||
=====Hepatic insufficiency===== | |||
* The mean elimination half-life of toremifene was increased by less than twofold in 10 patients with hepatic impairment ([[cirrhosis]] or [[fibrosis]]) compared to subjects with normal hepatic function. The pharmacokinetics of N-demethyltoremifene were unchanged in these patients. Ten patients on anticonvulsants ([[phenobarbital]], [[clonazepam]], [[phenytoin]], and [[carbamazepine]]) showed a twofold increase in clearance and a decrease in the elimination half-life of toremifene. | |||
===== | =====Geriatric patients===== | ||
* The pharmacokinetics of toremifene were studied in 10 healthy young males and 10 elderly females following a single 120 mg dose under fasting conditions. Increases in the elimination half-life (4.2 versus 7.2 days) and the volume of distribution (457 versus 627 L) of toremifene were seen in the elderly females without any change in clearance or AUC. The median ages in the three controlled studies ranged from 60 to 66 years. No significant age-related differences in Toremifene effectiveness or safety were noted. | |||
=====Food===== | |||
* The rate and extent of absorption of Toremifene are not influenced by food; thus Toremifene may be taken with or without food. | |||
=====Race===== | |||
* The pharmacokinetics of toremifene in patients of different races has not been studied. Fourteen percent of patients in the North American Study were non-Caucasian. No significant race-related differences in Toremifene effectiveness or safety were noted. | |||
===== | <!--Nonclinical Toxicology--> | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, and Impairment of Fertility===== | |||
* Conventional carcinogenesis studies in rats at doses of 0.12 to 12 mg/kg/day (approximately 1/50 to 2 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for up to 2 years did not show evidence of carcinogenicity. Studies in mice at doses of 1.0 to 30.0 mg/kg/day (approximately 1/15 to 2 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for up to 2 years revealed increased incidence of ovarian and testicular tumors and increased incidence of osteoma and osteosarcoma. The significance of the mouse findings is uncertain because of the different role of estrogens in mice and the estrogenic effect of toremifene in mice. An increased incidence of ovarian and testicular tumors in mice has also been observed with other human estrogen agonists/antagonists that have primarily estrogenic activity in mice. Endometrial hyperplasia of the uterus was observed in monkeys following 52 weeks of treatment at ≥1 mg/kg and in dogs following 16 weeks of treatment at ≥3 mg/kg with toremifene (approximately 1/3 and 1.4 times, respectively, the daily maximum recommended human dose of 60 mg, on a mg/m2 basis). | |||
* Toremifene has not been shown to be mutagenic in in vitro tests (Ames and E. coli bacterial tests). Toremifene is clastogenic in vitro (chromosomal aberrations and micronuclei formation in human lymphoblastoid MCL-5 cells) and in vivo (chromosomal aberrations in rat hepatocytes). | |||
* Toremifene produced impairment of fertility and conception in male and female rats at doses ≥25.0 and 0.14 mg/kg/day, respectively (approximately 4 times and 1/50 the daily maximum recommended human dose of 60 mg, on a mg/m2 basis). At these doses, sperm counts, fertility index, and conception rate were reduced in males with atrophy of seminal vesicles and prostate. In females, fertility and reproductive indices were markedly reduced with increased pre- and post-implantation loss. In addition, offspring of treated rats exhibited depressed reproductive indices. Toremifene produced ovarian atrophy in dogs administered doses ≥3 mg/kg/day (approximately 1.5 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for 16 weeks. Cystic ovaries and reduction in endometrial stromal cellularity were observed in monkeys at doses ≥1 mg/kg/day (about 1/3 the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for 52 weeks. | |||
= | <!--Clinical Studies--> | ||
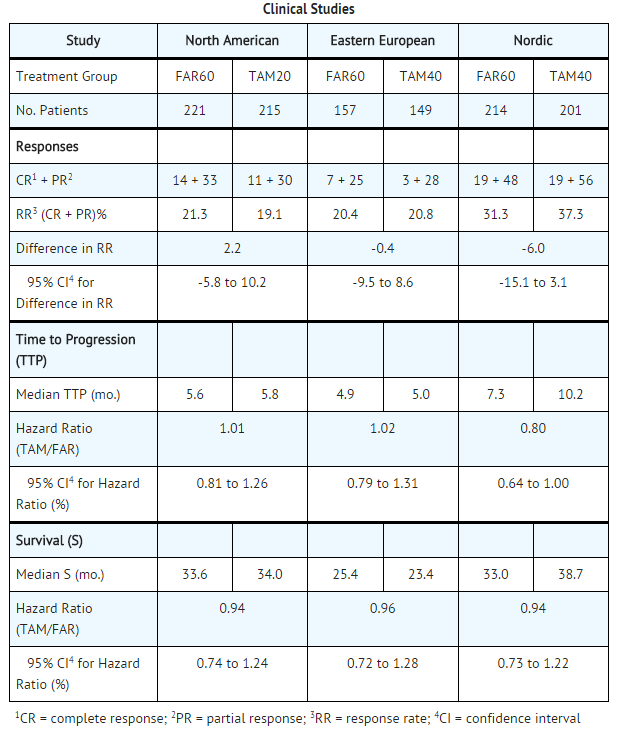
|clinicalStudies=* Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of Toremifene for the treatment of breast cancer in postmenopausal women. The patients were randomized to parallel groups receiving Toremifene 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively. The studies included postmenopausal patients with [[estrogen-receptor]] ([[ER]]) positive or [[estrogen-receptor]] ([[ER]]) unknown metastatic breast cancer. The patients had at least one measurable or evaluable lesion. The primary efficacy variables were response rate (RR) and time to progression (TTP). Survival (S) was also determined. Ninety-five percent confidence intervals (95% CI) were calculated for the difference in RR between FAR60 and TAM groups and the hazard ratio (relative risk for an unfavorable event, such as disease progression or death) between TAM and FAR60 for TTP and S. | |||
* Two of the 3 studies showed similar results for all effectiveness endpoints. However, the Nordic Study showed a longer time to progression for tamoxifen (see table). | |||
: [[File:Toremefine CS.png|none|500px]] | |||
* The high-dose groups, toremifene 200 mg daily in the North American Study and 240 mg daily in the Eastern European Study, were not superior to the lower toremifene dose groups, with response rates of 22.6% and 28.7%, median times to progression of 5.6 and 6.1 months, and median survivals of 30.1 and 23.8 months, respectively. The median treatment duration in the three pivotal studies was 5 months (range 4.2-6.3 months). | |||
<!--How Supplied--> | |||
|howSupplied=* Toremifene Tablets, containing toremifene citrate in an amount equivalent to 60 mg of toremifene, are round, convex, unscored, uncoated, and white, or almost white. | |||
* Toremifene Tablets are identified with TO 60 embossed on one side. | |||
* Toremifene Tablets are available as: | |||
: NDC 11399-005-30 bottles of 30 | |||
: NDC 11399-005 -01 bottles of 100 | |||
: NDC 11399-005-07 samples of 7 | |||
|storage=* Store at 25°C (77°F). | |||
* Excursions permitted to 15-30°C (59-86°F) | |||
|packLabel=====PRINCIPAL DISPLAY PANEL==== | |||
=====Principal Display Panel – Bottle Label===== | |||
NDC 11399-005-30 | |||
Toremifene® | |||
(toremifene citrate) | |||
Tablets | |||
30 TABLETS | |||
Rx Only | |||
60 mg | |||
GTx™ | |||
: [[File:Toremifine PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
=====Principal Display Panel – Bottle Label===== | |||
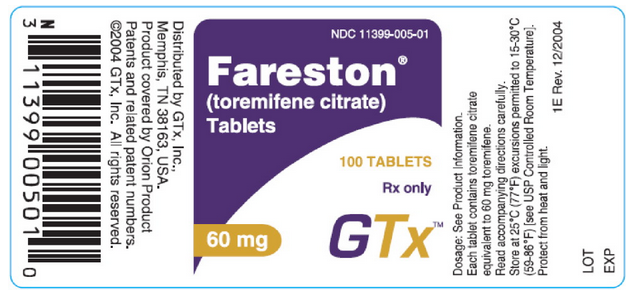
NDC 11399-005-01 | |||
Toremifene® | |||
(toremifene citrate) | |||
Tablets | |||
100 TABLETS | |||
Rx Only | |||
60 mg | |||
GTx™ | |||
: [[File:Toremifine PDP 2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
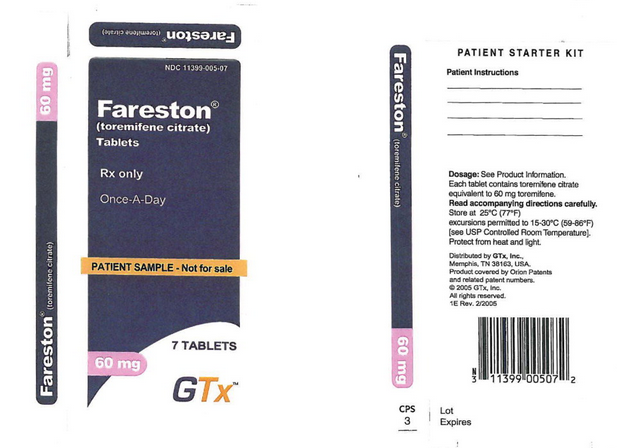
===== | =====Principal Display Panel – Sample Label===== | ||
11399-005-07 | |||
Toremifene® | |||
(toremifene citrate) | |||
Tablets | |||
Rx only | |||
Once-A-Day | |||
PATIENT SAMPLE – Not for sale | |||
7 Tablets | |||
60 mg | |||
GTx™ | |||
| | : [[File:Toremifine PDP 3.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
==== | ====Ingredients and Appearance==== | ||
: [[File:Toremifine Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
= | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=* Vaginal bleeding has been reported in patients using Toremifene. Patients should be informed about this and instructed to contact their physician if such bleeding occurs. | |||
* | * Toremifene may harm the fetus and increase the risk for pregnancy loss [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)]. | ||
* Premenopausal women using Toremifene should use nonhormonal contraception during treatment and should be apprised of the potential hazard to the fetus should pregnancy occur . | |||
* Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia and instructed to contact their physician for further assessment if such signs or symptoms occur. | |||
* Patients who must take medications known to prolong the QT interval, or potent CYP3A4 inhibitors, should be informed of the effect of toremifene on QT interval. Toremifene has been shown to prolong the QTc interval in a dose-related manner. | |||
* Specific interactions with foods that inhibit CYP3A4, including grapefruit juice, have not been studied but may increase toremifene concentrations. Patients should avoid grapefruit products and other foods that are known to inhibit CYP3A4 during Toremifene treatment. | |||
* Certain other medicines, including over-the-counter medications or herbal supplements (such as St. John's Wort) and toremifene, can reduce concentrations of coadministered drugs | |||
|alcohol=* Alcohol-Toremifene Citrate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol=* Alcohol- | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* Toremifene®<ref>{{Cite web | title = Toremifene Citrate | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a6ff9761-7442-4bff-9e50-858c03e27fd0}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Chemotherapeutic agents]] | |||
Latest revision as of 18:44, 6 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: QT PROLONGATION :
See full prescribing information for complete Boxed Warning.
WARNING: QT PROLONGATION :
|
Overview
Toremifene is an antiestrogen antineoplasic agent that is FDA approved for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hot flashes, sweating, nausea and vaginal discharge.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Toremifene® is an estrogen agonist/antagonist indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors.
Dosage
- The dosage of Toremifene is 60 mg, once daily, orally. Treatment is generally continued until disease progression is observed.
DOSAGE FORMS AND STRENGTHS
- Tablet is 60 mg, round, convex, unscored, uncoated, and white, or almost white, identified with TO 60 embossed on one side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Toremifene Citrate in adult patients.
Non–Guideline-Supported Use
- Fracture of vertebral column; Prophylaxis - Prostate cancer, Receiving androgen deprivation therapy[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Toremifene Citrate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Toremifene Citrate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Toremifene Citrate in pediatric patients.
Contraindications
Hypersensitivity to the Drug
- Toremifene is contraindicated in patients with known hypersensitivity to the drug.
QT Prolongation, Hypokalemia, Hypomagnesemia
- Toremifene should not be prescribed to patients with congenital/acquired QT prolongation (long QT syndrome), uncorrected hypokalemia, or uncorrected hypomagnesemia.
Warnings
|
WARNING: QT PROLONGATION :
See full prescribing information for complete Boxed Warning.
WARNING: QT PROLONGATION :
|
Prolongation of the QT Interval
- Toremifene has been shown to prolong the QTc interval in a dose- and concentration-related manner. Prolongation of the QT interval can result in a type of ventricular tachycardia called Torsade de pointes, which may result in syncope, seizure, and/or death.
- Toremifene should be avoided in patients with long QT syndrome. Caution should be exercised in patients with congestive heart failure, hepatic impairment and electrolyte abnormalities. Hypokalemia or hypomagnesemia must be corrected prior to initiating toremifene and these electrolytes should be monitored periodically during therapy. Drugs that prolong the QT interval should be avoided. In patients at increased risk, electrocardiograms (ECGs) should be obtained at baseline and as clinically indicated.
Hypercalcemia and Tumor Flare
- As with other antiestrogens, hypercalcemia and tumor flare have been reported in some breast cancer patients with bone metastases during the first weeks of treatment with Toremifene. Tumor flare is a syndrome of diffuse musculoskeletal pain and erythema with increased size of tumor lesions that later regress. It is often accompanied by hypercalcemia. Tumor flare does not imply failure of treatment or represent tumor progression. If hypercalcemia occurs, appropriate measures should be instituted and, if hypercalcemia is severe, Toremifene treatment should be discontinued.
Tumorigenicity
- Since most toremifene trials have been conducted in patients with metastatic disease, adequate data on the potential endometrial tumorigenicity of long-term treatment with Toremifene are not available. Endometrial hyperplasia has been reported. Some patients treated with Toremifene have developed endometrial cancer, but circumstances (short duration of treatment or prior antiestrogen treatment or premalignant conditions) make it difficult to establish the role of Toremifene. Endometrial hyperplasia of the uterus was observed in animals treated with toremifene.
General
- Patients with a history of thromboembolic diseases should generally not be treated with Toremifene. In general, patients with preexisting endometrial hyperplasia should not be given long-term Toremifene treatment. Patients with bone metastases should be monitored closely for hypercalcemia during the first weeks of treatment . Leukopenia and thrombocytopenia have been reported rarely; leukocyte and platelet counts should be monitored when using Toremifene in patients with leukopenia and thrombocytopenia.
Laboratory Tests
- Periodic complete blood counts, calcium levels, and liver function tests should be obtained.
Use in Pregnancy
- Based on its mechanism of action in humans and findings of increased pregnancy loss and fetal malformation in animal studies, Toremifene can cause fetal harm when administered to a pregnant woman. Toremifene caused embryo-fetal toxicities at maternal doses that were lower than the 60 mg daily recommended human dose on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women using Toremifene. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Women of Childbearing Potential
- Toremifene is indicated only in postmenopausal women. However, premenopausal women prescribed Toremifene should use effective non-hormonal contraception and should be apprised of the potential hazard to the fetus should pregnancy occur.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Trials Experience
- Adverse drug reactions are principally due to the antiestrogenic actions of Toremifene and typically occur at the beginning of treatment.
- The incidences of the following eight clinical toxicities were prospectively assessed in the North American Study. The incidence reflects the toxicities that were considered by the investigator to be drug related or possibly drug related.
- Approximately 1% of patients receiving Toremifene (n = 592) in the three controlled studies discontinued treatment as a result of adverse reactions (nausea and vomiting, fatigue, thrombophlebitis, depression, lethargy, anorexia, ischemic attack, arthritis, pulmonary embolism, and myocardial infarction).
- Serious adverse reactions occurring in at least 1% of patients receiving Toremifene in the three major trials are listed in the table below.
- Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted. The patients were randomized to parallel groups receiving Toremifene 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively
- Other adverse reactions included leukopenia and thrombocytopenia, skin discoloration or dermatitis, constipation, dyspnea, paresis, tremor, vertigo, pruritus, anorexia, reversible corneal opacity (corneal verticulata), asthenia, alopecia, depression, jaundice, and rigors.
- The incidence of AST elevations was greater in the 200 and 240 mg Toremifene dose arms than in the tamoxifen arms. Higher doses of Toremifene were also associated with an increase in nausea.
- Approximately 4% of patients were withdrawn for toxicity from the high-dose Toremifene treatment arms. Reasons for withdrawal included hypercalcemia, abnormal liver function tests, and one case each of toxic hepatitis, depression, dizziness, incoordination, ataxia, blurry vision, diffuse dermatitis, and a constellation of symptoms consisting of nausea, sweating, and tremor.
Postmarketing Experience
- The following adverse reactions were identified during post approval use of Toremifene. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Adverse reactions reported during post approval use of Toremifene have been consistent with clinical trial experience. The most frequently reported adverse reactions related to Toremifene use since market introduction include hot flash, sweating, nausea, and vaginal discharge.
Drug Interactions
Drugs that Decrease Renal Calcium Excretion
- Drugs that decrease renal calcium excretion, e.g., thiazide diuretics, may increase the risk of hypercalcemia in patients receiving Toremifene.
Agents that Prolong QT
- The administration of Toremifene with agents that have demonstrated QT prolongation as one of their pharmacodynamic effects should be avoided. Should treatment with any of these agents be required, it is recommended that therapy with Toremifene be interrupted. If interruption of treatment with Toremifene is not possible, patients who require treatment with a drug that prolongs QT should be closely monitored for prolongation of the QT interval. Agents generally accepted to prolong QT interval include Class 1A (e.g., quinidine, procainamide, disopyramide) and Class III (e.g., amiodarone, sotalol, ibutilide, dofetilide) antiarrhythmics; certain antipsychotics (e.g., thioridazine, haloperidol); certain antidepressants (e.g., venlafaxine, amitriptyline); certain antibiotics (e.g., erythromycin, clarithromycin, levofloxacin, ofloxacin); and certain anti-emetics (e.g., ondansetron, granisetron). In patients at increased risk, electrocardiograms (ECGs) should be obtained and patients monitored as clinically indicated.
Effect of Strong CYP3A4 Inducers on Toremifene
- Strong CYP3A4 enzyme inducers, such as dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, phenobarbital, St. John's Wort, lower the steady-state concentration of toremifene in serum.
Effect of Strong CYP3A4 Inhibitors on Toremifene
- In a study of 18 healthy subjects, 80 mg toremifene once daily coadministered with 200 mg of ketoconazole twice daily increased the toremifene Cmax and AUC by 1.4- and 2.9-fold, respectively. N-demethyltoremifene Cmax and AUC were reduced by 56% and 20%, respectively.
- The administration of Toremifene with agents that are strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole) increase the steady-state concentration in serum and should be avoided. Grapefruit juice may also increase plasma concentrations of toremifene and should be avoided. Should treatment with any of these agents be required, it is recommended that therapy with Toremifene be interrupted. If interruption of treatment with Toremifene is not possible, patients who require treatment with a drug that strongly inhibits CYP3A4 should be closely monitored for prolongation of the QT interval.
Effect of Toremifene on CYP3A4 Substrates
- In a study of 20 healthy subjects, 2 mg midazolam once daily (days 6 and 18) coadministered with toremifene as a 480 mg loading dose followed by 80 mg once daily for 16 days. Following coadministration on days 6 and 18 relevant increases in midazolam and α-hydroxymidazolam Cmax and AUC were not observed. Following coadministration on day 18 midazolam and α-hydroxymidazolam Cmax and AUC were reduced by less than 20%.
- Clinically relevant exposure changes in sensitive substrates due to inhibition or induction of CYP3A4 by toremifene appear unlikely.
Effect of Toremifene on CYP2C9 Substrates
- In a study of 20 healthy subjects, 500 mg tolbutamide once daily (days 7 and 19) coadministered with toremifene as a 480 mg loading dose followed by 80 mg once daily for 16 days. Following coadministration on days 7 and 19 plasma tolbutamide Cmax and AUC were increased by less than 30%. A reduction of similar magnitude was observed for hydroxytolbutamide and carboxytolbutamide Cmax and AUC.
- Toremifene is a weak inhibitor of CYP2C9. Concomitant use of CYP2C9 substrates with a narrow therapeutic index such as warfarin or phenytoin with Toremifene should be done with caution and requires careful monitoring (e.g., substrate concentrations (if possible), appropriate laboratory markers, and signs and symptoms of increased exposure).
Use in Specific Populations
Pregnancy
- Based on its mechanism of action in humans and findings of increased pregnancy loss and fetal malformation in animal studies, Toremifene can cause fetal harm when administered to a pregnant woman. Toremifene caused embryo-fetal toxicities at maternal doses that were lower than the 60 mg daily recommended human dose on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women using Toremifene. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- In animal studies, toremifene crossed the placenta and accumulated in the rodent fetus. Administration of toremifene to pregnant rats during organogenesis at doses of approximately 6% the daily maximum recommended human dose of 60 mg (on a mg/m2 basis) resulted in signs of maternal toxicity and increased preimplantation loss, increased resorptions, reduced fetal weight, and fetal anomalies. Fetal anomalies include malformation of limbs, incomplete ossification, misshapen bones, ribs/spine anomalies, hydroureter, hydronephrosis, testicular displacement, and subcutaneous edema. Maternal toxicity may have contributed to these adverse embryo-fetal effects. Similar embryo-fetal toxicities occurred in rabbits that received toremifene at doses approximately 40% the daily recommended human dose of 60 mg (on a mg/m2 basis). Findings in rabbits included increased preimplantation loss, increased resorptions, and fetal anomalies, including incomplete ossification and anencephaly.
- Animal doses resulting in embryo-fetal toxicities were ≥1.0 mg/kg/day in rats and ≥1.25 mg/kg/day in rabbits.
- In rodent models of fetal reproductive tract development, toremifene produced inhibition of uterine development in female pups similar to effects seen with diethylstilbestrol (DES) and tamoxifen. The clinical relevance of these changes is not known. Neonatal rodent studies have not been conducted to assess the potential for toremifene to cause other DES-like effects in offspring (i.e., vaginal adenosis). Vaginal adenosis in animals occurred following treatment with other drugs of this class and has been observed in women exposed to diethylstilbestrol in utero.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Toremifene Citrate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Toremifene Citrate during labor and delivery.
Nursing Mothers
- It is not known if toremifene is excreted in human milk. Toremifene is excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Toremifene, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- There is no indication for use of Toremifene in pediatric patients.
Geriatic Use
- The pharmacokinetics of toremifene were studied in 10 healthy young males and 10 elderly females following a single 120 mg dose under fasting conditions. Increases in the elimination half-life (4.2 versus 7.2 days) and the volume of distribution (457 versus 627 L) of toremifene were seen in the elderly females without any change in clearance or AUC.
- The median ages in the three controlled studies ranged from 60 to 66 years. No significant age-related differences in Toremifene effectiveness or safety were noted.
Gender
There is no FDA guidance on the use of Toremifene Citrate with respect to specific gender populations.
Race
- The pharmacokinetics of toremifene in patients of different races has not been studied.
- Fourteen percent of patients in the North American Study were non-Caucasian. No significant race-related differences in Toremifene effectiveness or safety were noted.
Renal Impairment
- The pharmacokinetics of toremifene and N-demethyltoremifene were similar in normals and in patients with impaired kidney function.
Hepatic Impairment
- The mean elimination half-life of toremifene was increased by less than twofold in 10 patients with hepatic impairment (cirrhosis or fibrosis) compared to subjects with normal hepatic function. The pharmacokinetics of N-demethyltoremifene were unchanged in these patients. Ten patients on anticonvulsants (phenobarbital, clonazepam, phenytoin, and carbamazepine) showed a twofold increase in clearance and a decrease in the elimination half-life of toremifene.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Toremifene Citrate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Toremifene Citrate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Hypokalemia or hypomagnesemia must be corrected prior to initiating toremifene and these electrolytes should be monitored periodically during therapy.
- Patients with bone metastases should be monitored closely for hypercalcemia during the first weeks of treatment
- leukocyte and platelet counts should be monitored when using Toremifene in patients with leukopenia and thrombocytopenia.
- Periodic complete blood counts, calcium levels, and liver function tests should be obtained.
IV Compatibility
There is limited information regarding IV Compatibility of Toremifene Citrate in the drug label.
Overdosage
- Lethality was observed in rats following single oral doses that were ≥1000 mg/kg (about 150 times the recommended human dose on a mg/m2 basis) and was associated with gastric atony/dilatation leading to interference with digestion and adrenal enlargement.
- Vertigo, headache, and dizziness were observed in healthy volunteer studies at a daily dose of 680 mg for 5 days. The symptoms occurred in two of the five subjects during the third day of the treatment and disappeared within 2 days of discontinuation of the drug. No immediate concomitant changes in any measured clinical chemistry parameters were found. In a study in postmenopausal breast cancer patients, toremifene 400 mg/m2/day caused dose-limiting nausea, vomiting, and dizziness, as well as reversible hallucinations and ataxia in one patient.
- Theoretically, overdose may be manifested as an increase of antiestrogenic effects, such as hot flashes; estrogenic effects, such as vaginal bleeding; or nervous system disorders, such as vertigo, dizziness, ataxia, and nausea. There is no specific antidote and the treatment is symptomatic.
Pharmacology
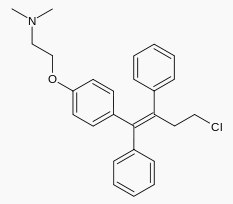
| |
Toremifene
| |
| Systematic (IUPAC) name | |
| 2-{4-[(1Z)-4-chloro-1,2-diphenyl-but-1-en-1-yl]phenoxy}-N,N-dimethylethanamine | |
| Identifiers | |
| CAS number | |
| ATC code | L02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 405.959 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | more than 99.5% |
| Metabolism | ? |
| Half life | 5 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Toremifene is a nonsteroidal triphenylethylene derivative. Toremifene binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities, depending upon the duration of treatment, animal species, gender, target organ, or endpoint selected. In general, however, nonsteroidal triphenylethylene derivatives are predominantly antiestrogenic in rats and humans and estrogenic in mice. In rats, toremifene causes regression of established dimethylbenzanthracene (DMBA)-induced mammary tumors. The antitumor effect of toremifene in breast cancer is believed to be mainly due to its antiestrogenic effects, i.e., its ability to compete with estrogen for binding sites in the cancer, blocking the growth-stimulating effects of estrogen in the tumor.
Structure
- Toremifene (toremifene citrate) Tablets for oral administration each contain 88.5 mg of toremifene citrate, which is equivalent to 60 mg toremifene.
- Toremifene is an estrogen agonist/antagonist. The chemical name of toremifene is: 2-{p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy}-N,N-dimethylethylamine citrate (1:1). The structural formula is:
and the molecular formula is C26H28ClNO • C6H8O7. The molecular weight of toremifene citrate is 598.10. The pKa is 8.0. Water solubility at 37˚C is 0.63 mg/mL and in 0.02N HCl at 37˚C is 0.38 mg/mL.
- Toremifene is available only as tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch.
Pharmacodynamics
- Toremifene causes a decrease in the estradiol-induced vaginal cornification index in some postmenopausal women, indicative of its antiestrogenic activity. Toremifene also has estrogenic activity as shown by decreases in serum gonadotropin concentrations (FSH and LH).
Effects on Cardiac Electrophysiology
- The effect of 20 mg, 80 mg, and 300 mg of toremifene on QT interval was evaluated in a double-blind, randomized study in healthy male subjects aged 18 to 45 years. The QT interval was measured at steady state of toremifene (Day 5 of dosing), including the time of peak plasma concentration (Tmax), at 13 time points (4 ECGs/time point) over 24 hours post dose in a time matched analysis. The 300 mg dose of toremifene (approximately five times the highest recommended dose 60 mg) was chosen because this dose produces exposure to toremifene that will cover the expected exposures that may result from potential drug interactions and hepatic impairment.
- Dose and concentration-related increases in the QTc interval and T wave changes were observed (see TABLE 1). These effects are believed to be caused by toremifene and N-demethyltoremifene. Toremifene had no effects on heart rate, PR and QRS interval duration
Pharmacokinetics
Absorption
- Toremifene is well absorbed after oral administration and absorption is not influenced by food. Peak plasma concentrations are obtained within 3 hours. Toremifene displays linear pharmacokinetics after single oral doses of 10 to 680 mg. After multiple dosing, dose proportionality was observed for doses of 10 to 400 mg. Steady state concentrations were reached in about 4-6 weeks.
Distribution
- Toremifene has an apparent volume of distribution of 580 L and binds extensively (>99.5%) to serum proteins, mainly albumin.
Metabolism
- Toremifene is extensively metabolized, principally by CYP3A4 to N-demethyltoremifene which is also antiestrogenic but with weak in vivo antitumor potency. Serum concentrations of N-demethyltoremifene are 2 to 4 times higher than toremifene at steady state.
- Following multiple dosing with toremifene in 20 healthy volunteers, plasma toremifene exposure was lower on Day 17 compared to Day 5 by approximately 14%. N-demethyltoremifene exposure was higher on Day 17 compared to Day 5 by approximately 80%. Based on these data and an in vitro induction study in human hepatocytes, auto-induction of CYP3A4 by toremifene is likely. The effect of auto-induction on efficacy was likely captured following prolonged dosing in the clinical studies.
Elimination
- The plasma concentration time profile of toremifene declines biexponentially after absorption with a mean distribution half-life of about 4 hours and an elimination half-life of about 5 days. Elimination half-lives of major metabolites, N-demethyltoremifene and (Deaminohydroxy) toremifene, were 6 and 4 days, respectively. Mean total clearance of toremifene was approximately 5 L/h. Toremifene is eliminated as metabolites primarily in the feces, with about 10% excreted in the urine during a 1-week period. Elimination of toremifene is slow, in part because of enterohepatic circulation.
Renal insufficiency
- The pharmacokinetics of toremifene and N-demethyltoremifene were similar in normals and patients with impaired kidney function.
Hepatic insufficiency
- The mean elimination half-life of toremifene was increased by less than twofold in 10 patients with hepatic impairment (cirrhosis or fibrosis) compared to subjects with normal hepatic function. The pharmacokinetics of N-demethyltoremifene were unchanged in these patients. Ten patients on anticonvulsants (phenobarbital, clonazepam, phenytoin, and carbamazepine) showed a twofold increase in clearance and a decrease in the elimination half-life of toremifene.
Geriatric patients
- The pharmacokinetics of toremifene were studied in 10 healthy young males and 10 elderly females following a single 120 mg dose under fasting conditions. Increases in the elimination half-life (4.2 versus 7.2 days) and the volume of distribution (457 versus 627 L) of toremifene were seen in the elderly females without any change in clearance or AUC. The median ages in the three controlled studies ranged from 60 to 66 years. No significant age-related differences in Toremifene effectiveness or safety were noted.
Food
- The rate and extent of absorption of Toremifene are not influenced by food; thus Toremifene may be taken with or without food.
Race
- The pharmacokinetics of toremifene in patients of different races has not been studied. Fourteen percent of patients in the North American Study were non-Caucasian. No significant race-related differences in Toremifene effectiveness or safety were noted.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Conventional carcinogenesis studies in rats at doses of 0.12 to 12 mg/kg/day (approximately 1/50 to 2 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for up to 2 years did not show evidence of carcinogenicity. Studies in mice at doses of 1.0 to 30.0 mg/kg/day (approximately 1/15 to 2 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for up to 2 years revealed increased incidence of ovarian and testicular tumors and increased incidence of osteoma and osteosarcoma. The significance of the mouse findings is uncertain because of the different role of estrogens in mice and the estrogenic effect of toremifene in mice. An increased incidence of ovarian and testicular tumors in mice has also been observed with other human estrogen agonists/antagonists that have primarily estrogenic activity in mice. Endometrial hyperplasia of the uterus was observed in monkeys following 52 weeks of treatment at ≥1 mg/kg and in dogs following 16 weeks of treatment at ≥3 mg/kg with toremifene (approximately 1/3 and 1.4 times, respectively, the daily maximum recommended human dose of 60 mg, on a mg/m2 basis).
- Toremifene has not been shown to be mutagenic in in vitro tests (Ames and E. coli bacterial tests). Toremifene is clastogenic in vitro (chromosomal aberrations and micronuclei formation in human lymphoblastoid MCL-5 cells) and in vivo (chromosomal aberrations in rat hepatocytes).
- Toremifene produced impairment of fertility and conception in male and female rats at doses ≥25.0 and 0.14 mg/kg/day, respectively (approximately 4 times and 1/50 the daily maximum recommended human dose of 60 mg, on a mg/m2 basis). At these doses, sperm counts, fertility index, and conception rate were reduced in males with atrophy of seminal vesicles and prostate. In females, fertility and reproductive indices were markedly reduced with increased pre- and post-implantation loss. In addition, offspring of treated rats exhibited depressed reproductive indices. Toremifene produced ovarian atrophy in dogs administered doses ≥3 mg/kg/day (approximately 1.5 times the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for 16 weeks. Cystic ovaries and reduction in endometrial stromal cellularity were observed in monkeys at doses ≥1 mg/kg/day (about 1/3 the daily maximum recommended human dose of 60 mg, on a mg/m2 basis) for 52 weeks.
Clinical Studies
- Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of Toremifene for the treatment of breast cancer in postmenopausal women. The patients were randomized to parallel groups receiving Toremifene 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively. The studies included postmenopausal patients with estrogen-receptor (ER) positive or estrogen-receptor (ER) unknown metastatic breast cancer. The patients had at least one measurable or evaluable lesion. The primary efficacy variables were response rate (RR) and time to progression (TTP). Survival (S) was also determined. Ninety-five percent confidence intervals (95% CI) were calculated for the difference in RR between FAR60 and TAM groups and the hazard ratio (relative risk for an unfavorable event, such as disease progression or death) between TAM and FAR60 for TTP and S.
- Two of the 3 studies showed similar results for all effectiveness endpoints. However, the Nordic Study showed a longer time to progression for tamoxifen (see table).
- The high-dose groups, toremifene 200 mg daily in the North American Study and 240 mg daily in the Eastern European Study, were not superior to the lower toremifene dose groups, with response rates of 22.6% and 28.7%, median times to progression of 5.6 and 6.1 months, and median survivals of 30.1 and 23.8 months, respectively. The median treatment duration in the three pivotal studies was 5 months (range 4.2-6.3 months).
How Supplied
- Toremifene Tablets, containing toremifene citrate in an amount equivalent to 60 mg of toremifene, are round, convex, unscored, uncoated, and white, or almost white.
- Toremifene Tablets are identified with TO 60 embossed on one side.
- Toremifene Tablets are available as:
- NDC 11399-005-30 bottles of 30
- NDC 11399-005 -01 bottles of 100
- NDC 11399-005-07 samples of 7
Storage
- Store at 25°C (77°F).
- Excursions permitted to 15-30°C (59-86°F)
Images
Drug Images
{{#ask: Page Name::Toremifene |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Bottle Label
NDC 11399-005-30
Toremifene®
(toremifene citrate)
Tablets
30 TABLETS
Rx Only
60 mg
GTx™
Principal Display Panel – Bottle Label
NDC 11399-005-01
Toremifene®
(toremifene citrate)
Tablets
100 TABLETS
Rx Only
60 mg
GTx™
Principal Display Panel – Sample Label
11399-005-07
Toremifene®
(toremifene citrate)
Tablets
Rx only
Once-A-Day
PATIENT SAMPLE – Not for sale
7 Tablets
60 mg
GTx™
Ingredients and Appearance
{{#ask: Label Page::Toremifene |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Vaginal bleeding has been reported in patients using Toremifene. Patients should be informed about this and instructed to contact their physician if such bleeding occurs.
- Toremifene may harm the fetus and increase the risk for pregnancy loss [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
- Premenopausal women using Toremifene should use nonhormonal contraception during treatment and should be apprised of the potential hazard to the fetus should pregnancy occur .
- Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia and instructed to contact their physician for further assessment if such signs or symptoms occur.
- Patients who must take medications known to prolong the QT interval, or potent CYP3A4 inhibitors, should be informed of the effect of toremifene on QT interval. Toremifene has been shown to prolong the QTc interval in a dose-related manner.
- Specific interactions with foods that inhibit CYP3A4, including grapefruit juice, have not been studied but may increase toremifene concentrations. Patients should avoid grapefruit products and other foods that are known to inhibit CYP3A4 during Toremifene treatment.
- Certain other medicines, including over-the-counter medications or herbal supplements (such as St. John's Wort) and toremifene, can reduce concentrations of coadministered drugs
Precautions with Alcohol
- Alcohol-Toremifene Citrate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Toremifene®[2]
Look-Alike Drug Names
There is limited information regarding Toremifene Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D; et al. (2010). "Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer". J Urol. 184 (4): 1316–21. doi:10.1016/j.juro.2010.06.022. PMC 3047407. PMID 20723926.
- ↑ "Toremifene Citrate".