Tongue cancer pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 50: | Line 50: | ||
*The changes in the adjoining mucosa | *The changes in the adjoining mucosa | ||
A fully developed tongue lesion appears as an exophytic bulky lesion that is gray to grayish-red and has a rough, shaggy, or papillomatous surface. | A fully developed tongue lesion appears as an exophytic bulky lesion that is gray to grayish-red and has a rough, shaggy, or papillomatous surface. | ||
===Microscopic Pathology=== | ===Microscopic Pathology=== | ||
*Microscopically, tongue cancers are broadly based and invasive through papillary fronds. | *Microscopically, tongue cancers are broadly based and invasive through papillary fronds. | ||
*Tongue cancer constitutes of highly differentiated squamous cells lacking frank cytologic criteria of malignancy with rare mitoses. The surface of the lesion is covered with compressed invaginating folds of [[keratin]] layers. A stroma-like inflammatory reaction and a blunt pushing margin may be seen. | *Tongue cancer constitutes of highly differentiated squamous cells lacking frank cytologic criteria of malignancy with rare mitoses. The surface of the lesion is covered with compressed invaginating folds of [[keratin]] layers. A stroma-like inflammatory reaction and a blunt pushing margin may be seen. | ||
[[File:Oral cancer (1) squamous cell carcinoma histopathology.jpg|30px|center|thumb|Microscopic picture of oral SCC, source: By No machine-readable author provided. KGH assumed (based on copyright claims). - No machine-readable source provided. Own work assumed (based on copyright claims)., CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=486166]] | |||
=== Classification === | === Classification === | ||
| Line 62: | Line 64: | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Revision as of 18:36, 30 November 2017
|
Tongue cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tongue cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Tongue cancer pathophysiology |
|
Risk calculators and risk factors for Tongue cancer pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Simrat Sarai, M.D. [2]
Overview
Genes involved in the pathogenesis of tongue cancer include TP53, c-myc, and erb-b1. On gross pathology, exophytic, ulcerative, and infiltarative growth patterns are characteristic findings of tongue cancer.[1]
Pathophysiology
Pathogenesis
- The two most common types of pre-cancerous conditions on the tongue are called leukoplakia and erythroplakia and they can usually be easily spotted by a dentist or dental hygienist. Leukoplakia and erythroplakia have the greatest potential for malignant transformation in tongue cancer. Leukoplakia is defined as a white patch of the mucosa that cannot be characterized clinically or pathologically as any other disease.
- Leukoplakia is considered a premalignant condition from the chronic irritation of the mucous membranes, resulting in increased rates of epithelial and connective tissue proliferation. Leukoplakia usually occurs after the age of 40 years, with the peak incidence before age 50 years. Leukoplakia is 2-3 times more common in men than in women.
- The rates of malignant transformation of leukoplakic lesions range from less than 1% to as high as 17.5%, averaging 4.5-6%. Erythroleukoplakia (leukoplakia erosiva) and nodular leukoplakia exhibit the highest rate of malignant transformation.
- Erythroplakia is defined as a red, velvety plaque found on the oral mucosa that cannot be ascribed to any other predetermined condition. No sex predilection is recognized in erythroplakia and it is rarely found on the tongue compared with other sites in the oral cavity. Erothroplakia is considered as the earliest sign of asymptomatic cancer by Mashberg.[1]
Tumor spread
Local spread
- in the early stages, is relatively predictable in tissues that have not been previously irradiated. It is influenced by local anatomical features.
- Lip SCC spreads superficially and then into deeper tissues.
- Floor of mouth SCC spreads superficially rather than in depth, being unlikely to invade into the mylohyoid muscle or the sublingual gland until a late stage.
- tumour involving the lateral margin of tongue, whether arising there directly or by superficial spread from the floor of mouth, tends to spread in depth.
- The intrinsic muscles of tongue run in small bundles in all directions such that invading tumour encounters some muscle running at right angles to the surface. The line of least resistance to tumour spread is therefore along these muscle bundles and into the tongue. Tumours of palate spread superficially rather than in depth and this is also true for more posterior tumours of the oropharynx.
Lymphatic spread
- Spread to local lymph nodes worsens the prognosis in oral and oropharyngeal cancer.
- The mechanism of spread from the primary site to lymph nodes is almost always by embolism. Permeation in lymphatics adjacent to tumours is uncommon and it is debatable if this spread extends as far as lymph nodes.
- Once tumour is present in the neck, however, spread between nodes may be embolic or by permeation.
- The lymph nodes in the neck are divided into levels. The lymphatic draianage from different head and neck sites is realtively predictable {1789}. Levels at high risk for metastasis from oral cavity SCC are Levels I, II and III, and to a lesser extent Level IV. Although Level II is the most frequently involved, some tumours spread to Level III or IV, with or without involvement of Level I.
- This has given rise to the concept of skip metastsasis. In reality the lymphatic drainage is complex and does not follow a regular sequence of levels of involvement in many patients {2817}.
- Bilateral spread to the neck is likely to occur from tumours involving the midline, especially tumours of posterior tongue or soft palate. Extracapsular spread of tumour involving lymph nodes is associated with a poor prognosis {2819}.
Haematogenous spread
- Until relatively recently, haematogenous spread of oral and oropharyngeal cancer has been regarded as less important than local and lymphatic spread.
- However, its importance is increasing as loco-regional control improves. Blood borne spread most often involves lung {754,1958}.
- The best predictor of the likelihood of this spread is involvement of the neck at multiple levels.
- This suggests that the route of entry of tumours into the circulation is most often via the large veins in the neck and that haematogenous spread is in effect tertiary spread following extracapsular spread from neck nodes.
Genetics
- Genes involved in the pathogenesis of tongue cancer include TP53, which is located on chromosome 17. The carcinogens in tobacco smoke, for example, increase the prevalence and spectrum of TP53 mutations {268}.
- Other oncogenes associated with squamous cell cancers of the tongue include c-myc and erb -b1.
- More than 50% of oropharyngeal carcinomas harbour integrated HPV DNA. The E6 and E7 viral oncoproteins bind and inactivate the TP53 and retinoblastoma gene products respectively, disengaging two of the more critical pathways involved in cell cycle regulation {2788}.
- local tumour recurrence reflects extension of genetically damaged cells beyond the clinical and microscopic boundaries of carcinoma to the margins of surgical resection {268,1626, 1983,2777}.
- Clonal genetic changes identical to those found in primary head and neck SCC have been identified in circulating plasma or serum, suggesting a mechanism for early cancer detection and tumour surveillance {1853}.
Gross pathology
Squamous cell carcinoma is the most common malignancy of the tongue. It typically has three gross morphologic growth patterns: exophytic, ulcerative, and infiltrative. The infiltrative and ulcerative are the types most commonly observed on the tongue.The macroscopic appearance of tongue cancer depends on the following:
- Duration of the lesion
- The amount of keratinization
- The changes in the adjoining mucosa
A fully developed tongue lesion appears as an exophytic bulky lesion that is gray to grayish-red and has a rough, shaggy, or papillomatous surface.
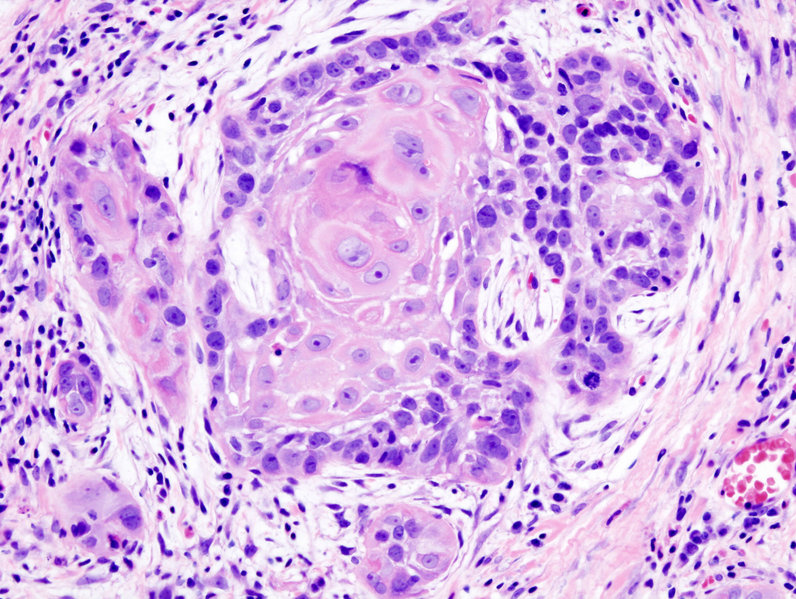
Microscopic Pathology
- Microscopically, tongue cancers are broadly based and invasive through papillary fronds.
- Tongue cancer constitutes of highly differentiated squamous cells lacking frank cytologic criteria of malignancy with rare mitoses. The surface of the lesion is covered with compressed invaginating folds of keratin layers. A stroma-like inflammatory reaction and a blunt pushing margin may be seen.
Classification
SCC is subdivided by the WHO into:
- Keratinizing type: Worst prognosis.
- Undifferentiated type: Intermediate prognosis, EBV association.
- Nonkeratinizing type: Good prognosis, EBV association.
References
- ↑ 1.0 1.1 A. Mashberg (1978). "Erythroplasia: the earliest sign of asymptomatic oral cancer". Journal of the American Dental Association (1939). 96 (4): 615–620. PMID 0273632. Unknown parameter
|month=ignored (help)