Stacking (chemistry)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
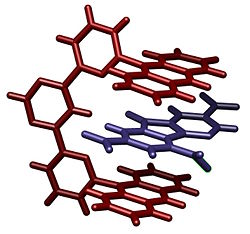
Stacking in supramolecular chemistry refers to a stacked arrangement of aromatic molecules, which interact through aromatic interactions. The most popular example of a stacked system is found for consecutive base pairs in DNA. Stacking also frequently occurs in enzymes where two relatively non-polar rings have overlapping pi orbitals. The exact nature of such interactions (electrostatic or nonelectrostatic) is a matter of debates.
Stacking in supramolecular chemistry
In supramolecular chemistry, an aromatic interaction (or π-π interaction) is a noncovalent interaction between organic compounds containing aromatic moieties. π-π interactions are caused by intermolecular overlapping of p-orbitals in π-conjugated systems, so they become stronger as the number of π-electrons increases. Other noncovalent interactions include hydrogen bonds, van der Waals forces, charge-transfer interactions, and dipole-dipole interactions.
π-π interactions act strongly on flat polycyclic aromatic hydrocarbons such as anthracene, triphenylene, and coronene because of the many delocalized π-electrons. This interaction, which is a bit stronger than other noncovalent interactions, plays an important role in various parts of supramolecular chemistry. For example, π-π interactions have a big influence on molecule-based crystal structures of aromatic compounds.
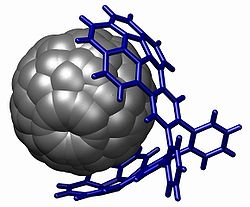
A powerful demonstration of stacking is found with the buckycatcher depicted below.[2] This molecular tweezer is based on two concave buckybowls with a perfect fit for one convex fullerene molecule. Complexation takes place simply by evaporating a toluene solution containing both compounds. In solution an association constant of 8600 M-1 is measured based on changes in NMR chemical shifts.
Stacking in biology
In DNA, pi stacking occurs between adjacent nucleotides and adds to the stability of the molecular structure. The nitrogenous bases of the nucleotides are made from either purine or pyrimidine rings, consisting of aromatic rings. Within the DNA molecule, the aromatic rings are positioned nearly perpendicular to the length of the DNA strands. Thus, the faces of the aromatic rings are arranged parallel to each other, allowing the bases to participate in aromatic interactions. Through aromatic interactions, the pi bonds, extending from atoms participating in double bonds, overlap with pi bonds of adjacent bases. This is a type of non-covalent chemical bond. Though a non-covalent bond is weaker than a covalent bond, the sum of all pi stacking interactions within the double-stranded DNA molecule creates a large net stabilizing energy.
Uses in materials
Many discotic liquid crystals can form columnar structures by π-π interactions. In addition, π-π interactions are an important factor in molecular self-assembly techniques in bottom-up nanotechnology.
Aromatic stacking interaction
Aromatic stacking interaction, sometimes called phenyl stacking, is a phenomenon in organic chemistry that affects aromatic compounds and functional groups. Because of especially strong Van der Waals bonding between the surfaces of flat aromatic rings, these groups in different molecules tend to arrange themselves like a stack of coins. This bonding behavior affects the properties of polymers as diverse as aramids, polystyrene, DNA, RNA, proteins, and peptides. The effect can be exploited in gas sensors to detect the presence of aromatic chemicals.
T-stacking
A related effect called T-stacking is often seen in proteins where the partially positively charged hydrogen atom of one aromatic system points perpendicular to the center of the aromatic plane of the other aromatic system.
See also
External links
References
- ↑ A. Petitjean, R. G. Khoury, N. Kyritsakas and J. M. Lehn (2004). "Dynamic Devices. Shape Switching and Substrate Binding in Ion-Controlled Nanomechanical Molecular Tweezers". J. Am. Chem. Soc. 126 (21): 6637–6647. doi:10.1021/ja031915r.
- ↑ 2.0 2.1 A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead (2007). "A Double Concave Hydrocarbon Buckycatcher". J. Am. Chem. Soc. 129 (13): 3842–3843. doi:10.1021/ja070616p.