Rhabdomyosarcoma pathophysiology: Difference between revisions
Jump to navigation
Jump to search
| Line 70: | Line 70: | ||
==Gross Pathology== | ==Gross Pathology== | ||
* | |||
[[File:Gross pathology of rhabdomyosarcoma.jpeg|thumb|none|300px|Gross feature of embryonal rhabdomyosarcoma of the nasopharynx (botryoid type) [https://www.humpath.com/rhinopharyngeal-embryonal, from flickr]]] | [[File:Gross pathology of rhabdomyosarcoma.jpeg|thumb|none|300px|Gross feature of embryonal rhabdomyosarcoma of the nasopharynx (botryoid type) [https://www.humpath.com/rhinopharyngeal-embryonal, from flickr]]] | ||
{| class="wikitable" | |||
|+ | |||
!Gross findings | |||
!Gross pathology | |||
|- | |||
| | |||
* Clusters of edematous and grape-like masses | |||
* Protrusion into lumen of hollow organs | |||
|[[File:Gross pathology of rhabdomyosarcoma.jpeg|thumb|none|300px|Gross feature of embryonal rhabdomyosarcoma of the nasopharynx (botryoid type) [https://www.humpath.com/rhinopharyngeal-embryonal, from flickr]]] | |||
|- | |||
| | |||
* Poorly circumscribed mass | |||
* white and infiltrative | |||
* soft or firm mass | |||
|[[File:Embryonal rhabdomyosarcoma.jpg|thumb|none|300px| Gross feature of orbital embryonal rhabdomyosarcoma [http://www.pathologyoutlines.com/topic/softtissueembryonalrhabdo.html Source: Contributed by Mark R. Wick, M.D., from Pathologyoutlines]]] | |||
|- | |||
| | |||
*Fleshy tumor | |||
*Tan gray color tumor | |||
|[[File:Alveolar rhabdomyosarcoma.jpg|thumb|none|300px| Gross pathology of alveolar rhabdomyosarcoma [http://www.pathologyoutlines.com/wick/softtissue/rhabdomyosarcomaalveolartypeleggross.jpg Source:Courtesy of Mark R. Wick, M.D., from Pathologyoutlines]]] | |||
|} | |||
[[File:Embryonal rhabdomyosarcoma.jpg|thumb|none|300px| Gross feature of orbital embryonal rhabdomyosarcoma [http://www.pathologyoutlines.com/topic/softtissueembryonalrhabdo.html Source: Contributed by Mark R. Wick, M.D., from Pathologyoutlines]]] | |||
* Gross pathology findings are: | * Gross pathology findings are: | ||
** Clusters of edematous and grape-like masses | ** Clusters of edematous and grape-like masses | ||
** Protrusion into lumen of hollow organs | ** Protrusion into lumen of hollow organs | ||
* Gross pathology findings are: | * Gross pathology findings are: | ||
** Poorly circumscribed mass | ** Poorly circumscribed mass | ||
Revision as of 18:14, 31 January 2019
|
Rhabdomyosarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rhabdomyosarcoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Rhabdomyosarcoma pathophysiology |
|
Risk calculators and risk factors for Rhabdomyosarcoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shadan Mehraban, M.D.[2]
Overview
Rhabdomyosarcoma arises from the skeletal muscle cells. Development of alveolar rhabdomyosarcoma is result of specific genetic mutations. The pathogenesis of rhabdomyosarcoma include t(2;13) and t(1;13) chromosomal translocations. The microscopic pathology of rhabdomyosarcoma depends on the histological subtype.
Pathophysiology
Histology
- The origin of rhabdomyosarcoma is straited muscle cells.[1]
- The presentation sites of rhabdomyosarcoma are:
- Head and neck (28%)
- Extremities (24%)
- Genitourinary tract (18%)
- Trunk (11%)
- Orbit (7%)
- Retroperitoneum (6%)
- Other sites such as bladder, vagina, nasopharynx, and middle ear (3%)
- Round or spindle-shaped muscle cells are present in embryonal rhabdomyosarcoma (ERMS).[2]
- Alveolar architecture with small round undifferentiated cells which are seprated by dense hyalinized fibrous septa are present in alveolar rhabdomyosarcoma (ARMS).
- Despite all muscle differentiation markers expression in ARMS, mature muscle characteristics is not seen in ARMS histology.
Pathogenesis
- The exact pathogenesis of rhabdomyosarcoma is unclear.[3]
- Distruption of skeletal muscle progenitor cell growth and related differentiation may cause rhabdomyosarcoma.[3]
- There is causal association between MET proto-oncogene and macrophage migration inhibitory factor (MIF).[4]
- It is thought that P53 is related to oncogenic transformation and tumor progression.[5]
Genetics
- According to microarray and targeted sequencing, following genomic characteristics are attributed to Rhabdomyosarcoma (RMS):[6][7][8][9][10][11]
- Loss of heterozygosity of 11p15.
- Mutations in TP53
- Mutations in NRAS
- Mutations in KRAS
- Mutations in HRAS
- Mutations in PIK3CA
- Mutations in CTNNB1
- Mutations in FGFR4
- Translocations in PAX3 or PAX7 genes with FOXO1
- Specific genetic changes in different types of rhabdomyosarcoma:
- Alveolar rhabdomyosarcoma:[12][13]
- Translocation of FKHR gene on chromosome 13 with the PAX3 gene on chromosome 2 or the PAX7 gene on chromosome 1
- PAX7 translocation occur in younger patients with longer event-free survival rate
- Embryonal rhabdomyosarcoma:[14]
- Particular gene changes are not clear
- loss of heterozygosity from the 11p15 region
- Hyperdiploid gene
- Lack of gene amplification
Associated conditions
Immunohistochemistry
- Through immunohistochemistry, muscle specific proteins can be identified in RMS.[2]
- RMS stains positive for following proteins:
- Plyclonal desmin (99%)
- Muscle-specific actin (95%)
- Myogenin (95%)
- Expressed more in ARMS rather than ERMS
- Associated with poorer prognosis
- Myoglobin (78%)
Transmission electron microscopy
- Transmission electron microscopy (TEM) can be used for poorly differentiated or undifferentiated tumors.[15][16]
- Prescence of following proteins are associated with TEM features of RMS:
- Myofilaments
- Desmin and actin filaments
- Myotubular intermediate filaments
- Rudimentary Z-band material
Reverse transcriptase polymerase chain reaction (RT-PCR)
Fluorescent in situ hybridization (FISH)
- By using RT-PCR and FISH, features of fusion proteins are identified in ARMS.[17]
Gross Pathology

| Gross findings | Gross pathology |
|---|---|
|
 |
|
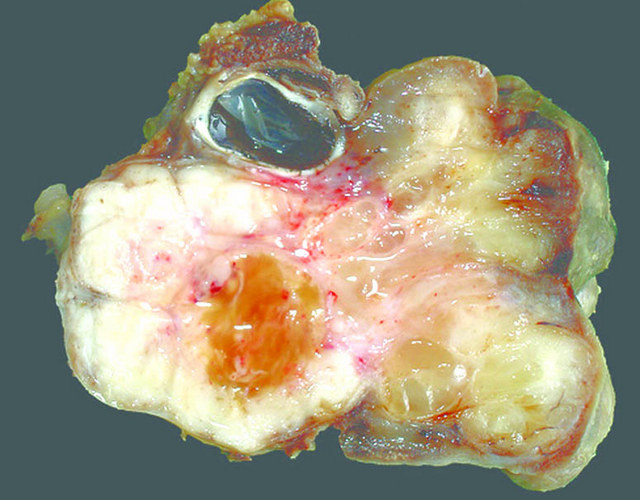 |
|
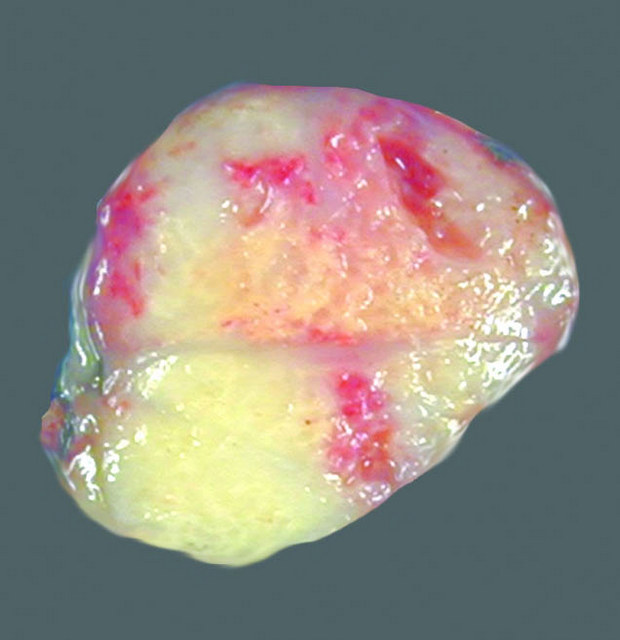 |

- Gross pathology findings are:
- Clusters of edematous and grape-like masses
- Protrusion into lumen of hollow organs
- Gross pathology findings are:
- Poorly circumscribed mass
- white and infiltrative
- soft or firm mass

- Gross pathology finding are:
- Fleshy tumor
- Tan gray color tumor
Microscopic Pathology
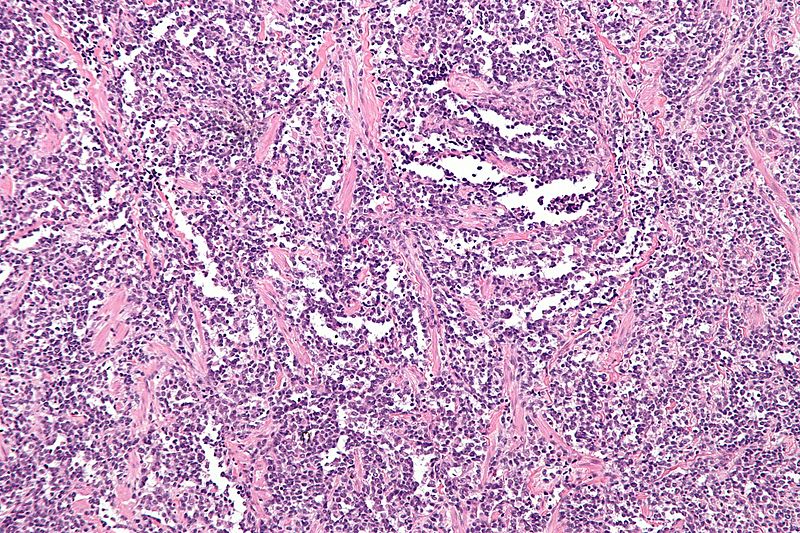
Alveolar Rhabdomyosarcoma
- The microscopic features of ARMS are:[18]
- Ovoid to round tumor cells are packed with fibrovascular septae
- Tumor cells have club-shaped appearance
- Tumor cells are divided by pseudo-alveolar spaces, slightly like pulmonary alveoli
- Rhabdomyoblasts are shed into pseudo-alveolar areas
- Presence of more than 50% alveolar components
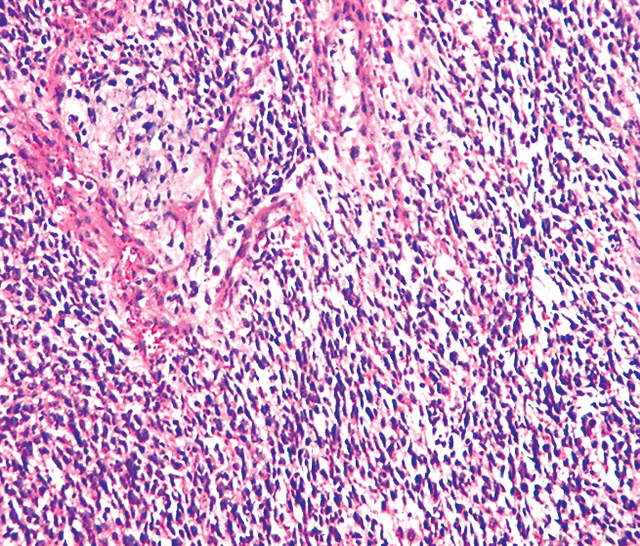
Embryonal Rhabdomyosarcoma
- The microscopic features of ERMS are:[19][14]
- Rhabdomyoblasts arranged in sheets and large nests
- Different stages of cell morphogenesis
- Positive stains are desmin, actin, myogenin, and MyoD1
- Infrequent intermixed fusiform cells
- No alveolar architectural pattern
- Typical rhabdomyoblast has eosinophilic cytoplasm with poorly formed myofilaments
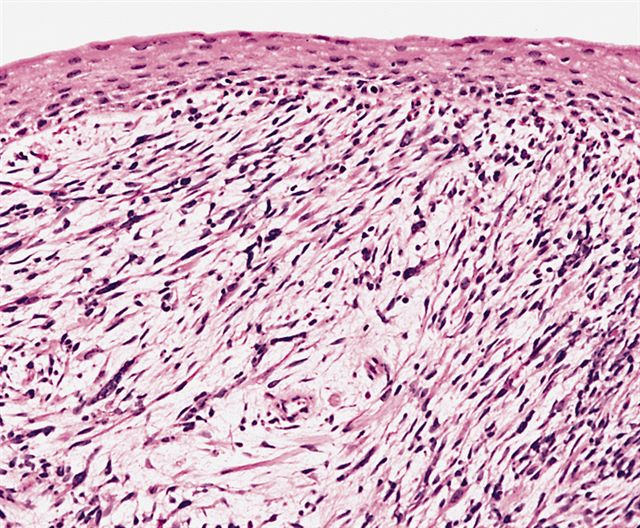
Botryoid Rhabdomyosarcoma
- The microscopic features of botryoid rhabdomyosarcoma:[20]
- Botryoid rhabdomyosarcoma grows beneath an epithelial surface
- Rhabdomyoblasts adhere to dense subepithelial which is called cambrium layer
- Malignant cells are found in myoxid stroma
Anaplastic Rhabdomyosarcoma
- The microscopic features of anaplastic rhabdomyosarcoma are:[21]
- Presence of large hyperchromatic nuclei
- Atypical bizarre mitotic features
- Threefold larger nuclear size
References
- ↑ Barr FG (1997). "Molecular genetics and pathogenesis of rhabdomyosarcoma". J Pediatr Hematol Oncol. 19 (6): 483–91. PMID 9407933.
- ↑ 2.0 2.1 Dias P, Chen B, Dilday B, Palmer H, Hosoi H, Singh S; et al. (2000). "Strong immunostaining for myogenin in rhabdomyosarcoma is significantly associated with tumors of the alveolar subclass". Am J Pathol. 156 (2): 399–408. doi:10.1016/S0002-9440(10)64743-8. PMC 1850049. PMID 10666368.
- ↑ 3.0 3.1 Taulli R, Scuoppo C, Bersani F, Accornero P, Forni PE, Miretti S; et al. (2006). "Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma". Cancer Res. 66 (9): 4742–9. doi:10.1158/0008-5472.CAN-05-4292. PMID 16651427.
- ↑ Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J; et al. (2010). "Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts". Mol Cancer Res. 8 (10): 1328–43. doi:10.1158/1541-7786.MCR-10-0288. PMC 2974061. PMID 20861157.
- ↑ Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H; et al. (2010). "Targeting wild-type and mutant p53 with small molecule CP-31398 blocks the growth of rhabdomyosarcoma by inducing reactive oxygen species-dependent apoptosis". Cancer Res. 70 (16): 6566–76. doi:10.1158/0008-5472.CAN-10-0942. PMC 2922473. PMID 20682800.
- ↑ Scrable H, Cavenee W, Ghavimi F, Lovell M, Morgan K, Sapienza C (1989). "A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting". Proc Natl Acad Sci U S A. 86 (19): 7480–4. PMC 298088. PMID 2798419.
- ↑ Taylor AC, Shu L, Danks MK, Poquette CA, Shetty S, Thayer MJ; et al. (2000). "P53 mutation and MDM2 amplification frequency in pediatric rhabdomyosarcoma tumors and cell lines". Med Pediatr Oncol. 35 (2): 96–103. PMID 10918230.
- ↑ Stratton MR, Fisher C, Gusterson BA, Cooper CS (1989). "Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction". Cancer Res. 49 (22): 6324–7. PMID 2680062.
- ↑ Shukla N, Ameur N, Yilmaz I, Nafa K, Lau CY, Marchetti A; et al. (2012). "Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways". Clin Cancer Res. 18 (3): 748–57. doi:10.1158/1078-0432.CCR-11-2056. PMC 3271129. PMID 22142829.
- ↑ Taylor JG, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K; et al. (2009). "Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models". J Clin Invest. 119 (11): 3395–407. doi:10.1172/JCI39703. PMC 2769177. PMID 19809159.
- ↑ Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS (1993). "Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma". Nat Genet. 3 (2): 113–7. doi:10.1038/ng0293-113. PMID 8098985.
- ↑ Missiaglia E, Williamson D, Chisholm J, Wirapati P, Pierron G, Petel F; et al. (2012). "PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification". J Clin Oncol. 30 (14): 1670–7. doi:10.1200/JCO.2011.38.5591. PMID 22454413.
- ↑ Cao L, Yu Y, Bilke S, Walker RL, Mayeenuddin LH, Azorsa DO; et al. (2010). "Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer". Cancer Res. 70 (16): 6497–508. doi:10.1158/0008-5472.CAN-10-0582. PMC 2922412. PMID 20663909.
- ↑ 14.0 14.1 Barr FG (1997). "Molecular genetics and pathogenesis of rhabdomyosarcoma". J Pediatr Hematol Oncol. 19 (6): 483–91. PMID 9407933.
- ↑ Hicks J, Flaitz C (2002). "Rhabdomyosarcoma of the head and neck in children". Oral Oncol. 38 (5): 450–9. PMID 12110339.
- ↑ Parham DM (2001). "Pathologic classification of rhabdomyosarcomas and correlations with molecular studies". Mod Pathol. 14 (5): 506–14. doi:10.1038/modpathol.3880339. PMID 11353062.
- ↑ Helman LJ, Meltzer P (2003). "Mechanisms of sarcoma development". Nat Rev Cancer. 3 (9): 685–94. doi:10.1038/nrc1168. PMID 12951587.
- ↑ Hostein I, Andraud-Fregeville M, Guillou L, Terrier-Lacombe MJ, Deminière C, Ranchère D; et al. (2004). "Rhabdomyosarcoma: value of myogenin expression analysis and molecular testing in diagnosing the alveolar subtype: an analysis of 109 paraffin-embedded specimens". Cancer. 101 (12): 2817–24. doi:10.1002/cncr.20711. PMID 15536621.
- ↑ Qualman SJ, Coffin CM, Newton WA, Hojo H, Triche TJ, Parham DM; et al. (1998). "Intergroup Rhabdomyosarcoma Study: update for pathologists". Pediatr Dev Pathol. 1 (6): 550–61. PMID 9724344.
- ↑ Neha B, Manjunath AP, Girija S, Pratap K (2015). "Botryoid Rhabdomyosarcoma of the Cervix: Case report with review of the literature". Sultan Qaboos Univ Med J. 15 (3): e433–7. doi:10.18295/squmj.2015.15.03.022. PMC 4554283. PMID 26357564.
- ↑ Hettmer S, Archer NM, Somers GR, Novokmet A, Wagers AJ, Diller L; et al. (2014). "Anaplastic rhabdomyosarcoma in TP53 germline mutation carriers". Cancer. 120 (7): 1068–75. doi:10.1002/cncr.28507. PMC 4173134. PMID 24382691.