Rhabdomyosarcoma pathophysiology: Difference between revisions
No edit summary |
(,) |
||
| Line 9: | Line 9: | ||
===Histology=== | ===Histology=== | ||
* The origin of rhabdomyosarcoma is muscle cells.<ref name="pmid9407933">{{cite journal| author=Barr FG| title=Molecular genetics and pathogenesis of rhabdomyosarcoma. | journal=J Pediatr Hematol Oncol | year= 1997 | volume= 19 | issue= 6 | pages= 483-91 | pmid=9407933 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9407933 }} </ref> | * The origin of rhabdomyosarcoma is straited muscle cells.<ref name="pmid9407933">{{cite journal| author=Barr FG| title=Molecular genetics and pathogenesis of rhabdomyosarcoma. | journal=J Pediatr Hematol Oncol | year= 1997 | volume= 19 | issue= 6 | pages= 483-91 | pmid=9407933 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9407933 }} </ref> | ||
* The presentation sites of rhabdomyosarcoma are: | * The presentation sites of rhabdomyosarcoma are: | ||
** Head and neck (28%) | ** Head and neck (28%) | ||
| Line 18: | Line 18: | ||
**Retroperitoneum (6%) | **Retroperitoneum (6%) | ||
**Other sites such as bladder, vagina, nasopharynx, and middle ear (3%) | **Other sites such as bladder, vagina, nasopharynx, and middle ear (3%) | ||
===Pathogenesis=== | |||
*The exact pathogenesis of rhabdomyosarcoma is unclear.<ref name="pmid16651427">{{cite journal| author=Taulli R, Scuoppo C, Bersani F, Accornero P, Forni PE, Miretti S et al.| title=Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. | journal=Cancer Res | year= 2006 | volume= 66 | issue= 9 | pages= 4742-9 | pmid=16651427 | doi=10.1158/0008-5472.CAN-05-4292 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16651427 }} </ref> | |||
*Distruption of skeletal muscle progenitor cell growth and related differentiation may cause rhabdomyosarcoma.<ref name="pmid20861157">{{cite journal| author=Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J et al.| title=Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. | journal=Mol Cancer Res | year= 2010 | volume= 8 | issue= 10 | pages= 1328-43 | pmid=20861157 | doi=10.1158/1541-7786.MCR-10-0288 | pmc=2974061 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20861157 }} </ref> | |||
*There is causal association between MET proto-oncogene and macrophage migration inhibitory factor (MIF).<ref name="pmid20682800">{{cite journal| author=Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H et al.| title=Targeting wild-type and mutant p53 with small molecule CP-31398 blocks the growth of rhabdomyosarcoma by inducing reactive oxygen species-dependent apoptosis. | journal=Cancer Res | year= 2010 | volume= 70 | issue= 16 | pages= 6566-76 | pmid=20682800 | doi=10.1158/0008-5472.CAN-10-0942 | pmc=2922473 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20682800 }} </ref> | |||
*It is thought that P53 is related to oncogenic transformation and tumor progression. | |||
===Genetics=== | ===Genetics=== | ||
Specific genetic abnormalities have been identified, that are specific for alveolar rhabdomyosarcomas. They include t(2;13) and t(1;13) [[Chromosomal translocation|chromosomal translocations]] resulting in ''PAX3-FKHR'' and ''PAX7-FKHR'' gene fusions. | Specific genetic abnormalities have been identified, that are specific for alveolar rhabdomyosarcomas. They include t(2;13) and t(1;13) [[Chromosomal translocation|chromosomal translocations]] resulting in ''PAX3-FKHR'' and ''PAX7-FKHR'' gene fusions. | ||
Revision as of 15:22, 30 January 2019
|
Rhabdomyosarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rhabdomyosarcoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Rhabdomyosarcoma pathophysiology |
|
Risk calculators and risk factors for Rhabdomyosarcoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shadan Mehraban, M.D.[2]
Overview
Rhabdomyosarcoma arises from the skeletal muscle cells. Development of alveolar rhabdomyosarcoma is result of specific genetic mutations. The pathogenesis of rhabdomyosarcoma include t(2;13) and t(1;13) chromosomal translocations. The microscopic pathology of rhabdomyosarcoma depends on the histological subtype.
Pathophysiology
Histology
- The origin of rhabdomyosarcoma is straited muscle cells.[1]
- The presentation sites of rhabdomyosarcoma are:
- Head and neck (28%)
- Extremities (24%)
- Genitourinary tract (18%)
- Trunk (11%)
- Orbit (7%)
- Retroperitoneum (6%)
- Other sites such as bladder, vagina, nasopharynx, and middle ear (3%)
Pathogenesis
- The exact pathogenesis of rhabdomyosarcoma is unclear.[2]
- Distruption of skeletal muscle progenitor cell growth and related differentiation may cause rhabdomyosarcoma.[3]
- There is causal association between MET proto-oncogene and macrophage migration inhibitory factor (MIF).[4]
- It is thought that P53 is related to oncogenic transformation and tumor progression.
Genetics
Specific genetic abnormalities have been identified, that are specific for alveolar rhabdomyosarcomas. They include t(2;13) and t(1;13) chromosomal translocations resulting in PAX3-FKHR and PAX7-FKHR gene fusions.
Microscopic Pathology
Characteristic features on microscopic analysis are variable depending on the rhabdomyosarcoma subtype:[5]
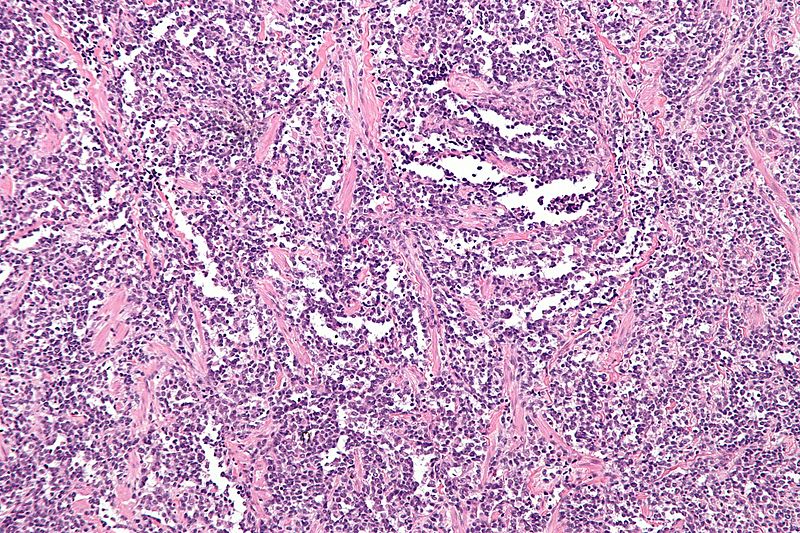
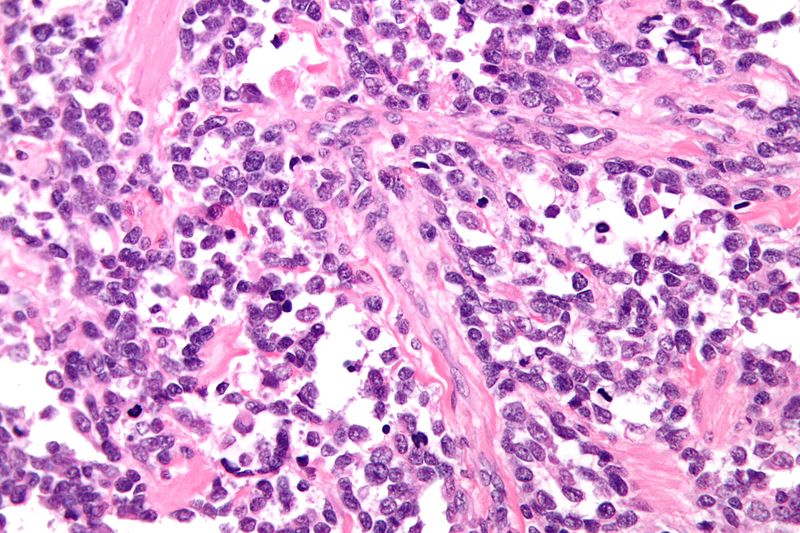
Alveolar Rhabdomyosarcoma
- Characterized by Alveolus-like pattern.
- Fibrous septae lined by tumor cells.
- Cells may "fall-off" the septa, i.e. be detached/scattered in the alveolus-like space.
- Space between fibrous septae may be filled with tumor, called solid variant of alveolar rhabdomyosarcoma.
- Rhabdomyoblasts: Essentially diagnostic cells with eccentric nucleus and moderate amount of intensely eosinophilic cytoplasm.
- Nuclear pleomorphism
- Mitoses
 |
 |
Embryonal Rhabdomyosarcoma
- Randomly arranged small cells
- Myxoid matrix
- Strap cells: Tadpole like morphology
- Rhabdomyoblasts: Essentially diagnostic cells with eccentric nucleus and moderate amount of intensely eosinophilic cytoplasm
Botryoid Rhabdomyosarcoma
- Malignant cells in an abundant myxoid stroma.
- Non-proliferating layer deep to the surface called "Cambium layer".
- Cambium layer is defined as cellular region deep to epithelial component.
Spindlecell Rhabdomyosarcoma
- Vesicular growth pattern
- Spindle cells
References
- ↑ Barr FG (1997). "Molecular genetics and pathogenesis of rhabdomyosarcoma". J Pediatr Hematol Oncol. 19 (6): 483–91. PMID 9407933.
- ↑ Taulli R, Scuoppo C, Bersani F, Accornero P, Forni PE, Miretti S; et al. (2006). "Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma". Cancer Res. 66 (9): 4742–9. doi:10.1158/0008-5472.CAN-05-4292. PMID 16651427.
- ↑ Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J; et al. (2010). "Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts". Mol Cancer Res. 8 (10): 1328–43. doi:10.1158/1541-7786.MCR-10-0288. PMC 2974061. PMID 20861157.
- ↑ Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H; et al. (2010). "Targeting wild-type and mutant p53 with small molecule CP-31398 blocks the growth of rhabdomyosarcoma by inducing reactive oxygen species-dependent apoptosis". Cancer Res. 70 (16): 6566–76. doi:10.1158/0008-5472.CAN-10-0942. PMC 2922473. PMID 20682800.
- ↑ "librepathology".