Rabies: Difference between revisions
(→Causes) |
(→Cause) |
||
| Line 27: | Line 27: | ||
==[[Rabies causes|Causes]]== | ==[[Rabies causes|Causes]]== | ||
==Cause== | ===Cause=== | ||
[[File:Rabies Virus EM PHIL 1876.JPG|thumb|[[Transmission electron microscopy|TEM]] [[micrograph]] with numerous rabies [[virion]]s (small, dark grey, rodlike particles) and [[Negri bodies]] (the larger [[pathognomonic]] cellular inclusions of rabies infection)]] | [[File:Rabies Virus EM PHIL 1876.JPG|thumb|[[Transmission electron microscopy|TEM]] [[micrograph]] with numerous rabies [[virion]]s (small, dark grey, rodlike particles) and [[Negri bodies]] (the larger [[pathognomonic]] cellular inclusions of rabies infection)]] | ||
Rabies is caused by a number of ''[[lyssavirus]]es'' including: [[rabies virus]] and [[Australian bat lyssavirus]].<ref>{{cite web|title=Rabies, Australian bat lyssavirus and other lyssaviruses|url=http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-rabies-consumer-info.htm|work=The Department of Health|accessdate=1 March 2014|date=Dec 2013}}</ref> | Rabies is caused by a number of ''[[lyssavirus]]es'' including: [[rabies virus]] and [[Australian bat lyssavirus]].<ref>{{cite web|title=Rabies, Australian bat lyssavirus and other lyssaviruses|url=http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-rabies-consumer-info.htm|work=The Department of Health|accessdate=1 March 2014|date=Dec 2013}}</ref> | ||
| Line 42: | Line 42: | ||
===Transmission=== | ===Transmission=== | ||
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. [[Bird]]s were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.<ref name=serological>{{cite journal |author=Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME |title=Serological survey for rabies antibodies in raptors from California |journal=J. Wildl. Dis. |volume=24 |issue=2 |pages=264–7 |date=April 1988 |pmid=3286906 |doi=10.7589/0090-3558-24.2.264 |url=http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-24.2.264}}</ref> Other bird species have been known to develop rabies [[antibody|antibodies]], a sign of infection, after feeding on rabies-infected mammals.<ref name="pmid16498885">{{cite journal | author = Gough PM, Jorgenson RD | title = Rabies antibodies in sera of wild birds | journal = Journal of Wildlife Diseases | volume = 12 | issue = 3 | pages = 392–5 | year = 1976 | pmid = 16498885 | doi = 10.7589/0090-3558-12.3.392| url =http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-12.3.392}}</ref><ref name=Owls>{{cite journal |author=Jorgenson RD, Gough PM |title=Experimental rabies in a great horned owl |journal=J. Wildl. Dis. |volume=12 |issue=3 |pages=444–7 |date=July 1976 |doi=10.7589/0090-3558-12.3.444 |url=http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-12.3.444}}</ref> | All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. [[Bird]]s were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.<ref name=serological>{{cite journal |author=Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME |title=Serological survey for rabies antibodies in raptors from California |journal=J. Wildl. Dis. |volume=24 |issue=2 |pages=264–7 |date=April 1988 |pmid=3286906 |doi=10.7589/0090-3558-24.2.264 |url=http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-24.2.264}}</ref> Other bird species have been known to develop rabies [[antibody|antibodies]], a sign of infection, after feeding on rabies-infected mammals.<ref name="pmid16498885">{{cite journal | author = Gough PM, Jorgenson RD | title = Rabies antibodies in sera of wild birds | journal = Journal of Wildlife Diseases | volume = 12 | issue = 3 | pages = 392–5 | year = 1976 | pmid = 16498885 | doi = 10.7589/0090-3558-12.3.392| url =http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-12.3.392}}</ref><ref name=Owls>{{cite journal |author=Jorgenson RD, Gough PM |title=Experimental rabies in a great horned owl |journal=J. Wildl. Dis. |volume=12 |issue=3 |pages=444–7 |date=July 1976 |doi=10.7589/0090-3558-12.3.444 |url=http://www.jwildlifedis.org/doi/abs/10.7589/0090-3558-12.3.444}}</ref> | ||
Revision as of 19:36, 7 August 2015
For patient information click here
| Rabies virus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Virus classification | ||||||||||
|
| Rabies | |
 | |
|---|---|
| Rabies virus |
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies On the Web |
|
American Roentgen Ray Society Images of Rabies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Michael Maddaleni, B.S.
Synonyms and keywords: Rabies virus infection
Overview
Historical Perspective
Pathophysiology
Causes
Cause
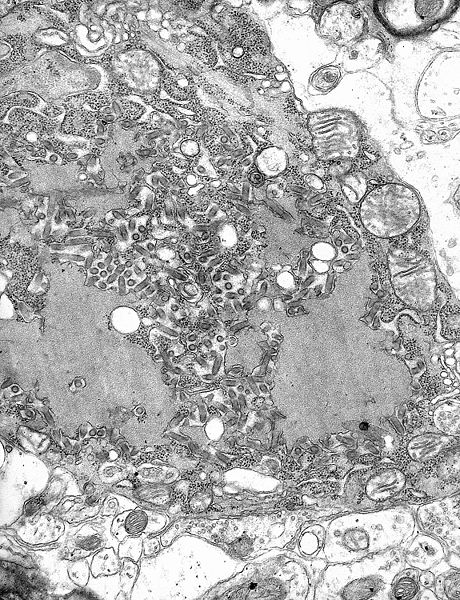
Rabies is caused by a number of lyssaviruses including: rabies virus and Australian bat lyssavirus.[1]
The rabies virus is the type species of the Lyssavirus genus, in the family Rhabdoviridae, order Mononegavirales. Lyssaviruses have helical symmetry, with a length of about 180 nm and a cross-section of about 75 nm.[2] These viruses are enveloped and have a single-stranded RNA genome with negative sense. The genetic information is packed as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and the viral RNA polymerase (L).[3]
Once within a muscle or nerve cell, the virus undergoes replication. The trimeric spikes on the exterior of the membrane of the virus interact with a specific cell receptor, the most likely one being the acetylcholine receptor, acetyl. The cellular membrane pinches in a procession known as pinocytosis and allows entry of the virus into the cell by way of an endosome. The virus then uses the acidic environment, which is necessary, of that endosome and binds to its membrane simultaneously, releasing its five proteins and single strand RNA into the cytoplasm.[4]
The L protein then transcribes five mRNA strands and a positive strand of RNA all from the original negative strand RNA using free nucleotides in the cytoplasm. These five mRNA strands are then translated into their corresponding proteins (P, L, N, G and M proteins) at free ribosomes in the cytoplasm. Some proteins require post-translative modifications. For example, the G protein travels through the rough endoplasmic reticulum, where it undergoes further folding, and is then transported to the Golgi apparatus, where a sugar group is added to it (glycosylation).[4]
Where there are enough proteins, the viral polymerase will begin to synthesize new negative strands of RNA from the template of the positive strand RNA. These negative strands will then form complexes with the N, P, L and M proteins and then travel to the inner membrane of the cell, where a G protein has embedded itself in the membrane. The G protein then coils around the N-P-L-M complex of proteins taking some of the host cell membrane with it, which will form the new outer envelope of the virus particle. The virus then buds from the cell.[4]
From the point of entry, the virus is neurotropic, traveling quickly along the neural pathways into the central nervous system. The virus usually first infects muscle cells close to the site of infection, where they are able to replicate without being 'noticed' by the host's immune system. Once enough virus has been replicated, they begin to bind to acetyl choline receptors (p75NR) at the neuromuscular junction. [5] The virus then travels through the nerve cell axon via retrograde transport, as its P protein interacts with dynein, a protein present in the cytoplasm of nerve cells. Once the virus reaches the cell body it travels rapidly to the Central Nervous System (CNS), replicating in motor neurons and eventually reaching to the brain. [6] After the brain is infected, the virus travels centrifugally to the peripheral and autonomic nervous systems, eventually migrating to the salivary glands, where it is ready to be transmitted to the next host.
Transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.[7] Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.[8][9]
The virus has also been adapted to grow in cells of poikilothermic ("cold-blooded") vertebrates.[10][11] Most animals can be infected by the virus and can transmit the disease to humans. Infected bats,[12][13] monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, dogs, mongooses (normally yellow mongoose)[14] and cats present the greatest risk to humans.
Rabies may also spread through exposure to infected domestic farm animals, groundhogs, weasels, bears, and other wild carnivorans. Small rodents, such as squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice, and lagomorphs such as rabbits and hares, are almost never found to be infected with rabies and are not known to transmit rabies to humans.[15] The Virginia opossum is resistant but not immune to rabies.[16]
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[17][18] The route of infection is usually, but not always, by a bite. In many cases, the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behavior.[19] This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts.
Transmission between humans is extremely rare. A few cases have been recorded through transplant surgery.[20] After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels along the afferent nerves toward the central nervous system.[21] During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis, the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.[22][23]
Differentiating Rabies from other Diseases
Epidemiology and Demographics
Risk Factors
Natural History, Complications and Prognosis
Diagnosis
History and Symptoms | Physical Examination | Laboratory Findings | CT | Other Diagnostic Studies
Treatment
Medical Therapy | Primary Prevention | Secondary Prevention | Cost-Effectiveness of Therapy | Future or Investigational Therapies
Case Studies
Related Chapters
External Links
- Centers for Disease Control and Prevention
- World Health Organization factsheet on Rabies
- World Health Organization factsheet on Rabies vaccine
ar:داء الكلب zh-min-nan:Siáu-káu-pēⁿ bg:Бяс cs:Vzteklina da:Hundegalskab de:Tollwut eo:Rabio eu:Amorru hr:Bjesnoća id:Rabies it:Rabbia he:כלבת la:Rabies lt:Pasiutligė nl:Hondsdolheid no:Rabies simple:Rabies ur:مرض کلب sk:Besnota sl:Steklina fi:Vesikauhu sv:Rabies th:โรคพิษสุนัขบ้า uk:Сказ wa:Må d' araedje
- ↑ "Rabies, Australian bat lyssavirus and other lyssaviruses". The Department of Health. Dec 2013. Retrieved 1 March 2014.
- ↑ Drew WL (2004). "Chapter 41: Rabies". In Ryan KJ, Ray CG (editors). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 597–600. ISBN 0-8385-8529-9.
- ↑ Finke S, Conzelmann KK (August 2005). "Replication strategies of rabies virus". Virus Res. 111 (2): 120–31. doi:10.1016/j.virusres.2005.04.004. PMID 15885837.
- ↑ 4.0 4.1 4.2
- ↑ http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1004348
- ↑
- ↑ Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME (April 1988). "Serological survey for rabies antibodies in raptors from California". J. Wildl. Dis. 24 (2): 264–7. doi:10.7589/0090-3558-24.2.264. PMID 3286906.
- ↑ Gough PM, Jorgenson RD (1976). "Rabies antibodies in sera of wild birds". Journal of Wildlife Diseases. 12 (3): 392–5. doi:10.7589/0090-3558-12.3.392. PMID 16498885.
- ↑ Jorgenson RD, Gough PM (July 1976). "Experimental rabies in a great horned owl". J. Wildl. Dis. 12 (3): 444–7. doi:10.7589/0090-3558-12.3.444.
- ↑ Wong, Derek. "Rabies". Wong's Virology. Retrieved 19 Mar 2009.
- ↑ Campbell, James B.; Charlton, K.M. (1988). Developments in Veterinary Virology: Rabies. Springer. p. 48. ISBN 0-89838-390-0.
- ↑ Pawan JL (1959). "The transmission of paralytic rabies in Trinidad by the vampire bat (Desmodus rotundus murinus Wagner". Caribbean Medical Journal. 21: 110–36. PMID 13858519.
- ↑ Pawan JL (1959). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Caribbean Medical Journal. 21: 137–56. PMID 14431118.
- ↑ Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". The Onderstepoort Journal of Veterinary Research. 60 (4): 379–87. PMID 7777324.
- ↑ "Rabies. Other Wild Animals: Terrestrial carnivores: raccoons, skunks and foxes". Centers for Disease Control and Prevention(CDC). Retrieved 2010-12-23.
- ↑ McRuer DL, Jones KD (May 2009). "Behavioral and nutritional aspects of the Virginian opossum (Didelphis virginiana)". The veterinary clinics of North America. Exotic animal practice. 12 (2): 217–36, viii. doi:10.1016/j.cvex.2009.01.007. PMID 19341950.
- ↑ The Merck Manual, 11th Edition (1983), p. 183
- ↑ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ↑ Turton, Jenny (2000). "Rabies: a killer disease". National Department of Agriculture.
- ↑ Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR (March 2005). "Transmission of rabies virus from an organ donor to four transplant recipients" (PDF). N Engl J Med. 352 (11): 1103–11. doi:10.1056/NEJMoa043018. PMID 15784663.
- ↑ Jackson, Alan C., Wunner, William H. (2002). Rabies. Academic Press. p. 290. ISBN 978-0-12-379077-4.
- ↑ Joanne Lynn, M.D. (October 1997) Transverse Myelitis: Symptoms, Causes and Diagnosis The Transverse Myelitis Association
- ↑ Larry Ernest Davis; Molly K. King; Jessica L. Schultz (15 June 2005). Fundamentals of neurologic disease. Demos Medical Publishing. p. 73. ISBN 978-1-888799-84-2.