Omacetaxine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Omacetaxine is a that is FDA approved for the {{{indicationType}}} of adult patients with chronic or accelerated phase chronic myeloid leukemia (CML) with resistance and/or intolerance to two or more tyrosine kinase inhibitors (TKI).. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Chronic myeloid leukemia
Omacetaxine is indicated for the treatment of adult patients with chronic or accelerated phase chronic myeloid leukemia (CML) with resistance and/or intolerance to two or more tyrosine kinase inhibitors (TKI).
Dosing Information
Induction Schedule
- The recommended starting schedule for induction is 1.25 mg/m2 administered subcutaneously twice daily at approximately 12 hour intervals for 14 consecutive days every 28 days, over a 28-day cycle. Cycles should be repeated every 28 days until patients achieve a hematologic response.
Maintenance Dosing
- The recommended maintenance schedule is 1.25 mg/m2 administered subcutaneously twice daily approximately 12 hour intervals for 7 consecutive days every 28 days, over a 28-day cycle. *Treatment should continue as long as patients are clinically benefiting from therapy.
Dose Adjustments and Modifications
Hematologic Toxicity:
- Omacetaxine treatment cycles may be delayed and/or the number of days of dosing during the cycle reduced for hematologic toxicities (e.g. neutropenia, thrombocytopenia) .
- Complete blood counts (CBCs) should be performed weekly during induction and initial maintenance cycles. After initial maintenance cycles, monitor CBCs every two weeks or as clinically indicated. If a patient experiences Grade 4 neutropenia (absolute neutrophil count (ANC) less than 0.5 x 109/L) or Grade 3 thrombocytopenia (platelet counts less than 50 x 109/L) during a cycle, delay starting the next cycle until ANC is greater than or equal to 1.0 x 109/L and platelet count is greater than or equal to 50 x 109/L. Also, for the next cycle, reduce the number of dosing days by 2 days (e.g. to 12 or 5 days).
Non-Hematologic Toxicity:
- Manage other clinically significant non-hematologic toxicity symptomatically. Interrupt and/or delay Omacetaxine until toxicity is resolved.
Reconstitution Instructions and Handling Precautions
- Omacetaxine should be prepared in a healthcare facility and must be reconstituted by a healthcare professional.
- Reconstitute Omacetaxine with one mL of 0.9% Sodium Chloride Injection, USP, prior to subcutaneous injection. After addition of the diluent, gently swirl until a clear solution is obtained. The lyophilized powder should be completely dissolved in less than one minute. The resulting solution is clear and colorless and contains 3.5 mg/mL Omacetaxine . Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
- Omacetaxine does not contain antimicrobial preservatives. Therefore care must be taken to ensure that the solution for injection is not contaminated during preparation.
- Omacetaxine is a cytotoxic drug. Follow special handling and disposal procedures. Wear protective eyewear and gloves during handling and administration of the product. Proper aseptic technique should be used. Avoid skin and eye contact. If Omacetaxine comes into contact with skin, immediately and thoroughly wash affected area with soap and water. If contact with the eyes occurs, thoroughly flush the eyes with water.
Storage Conditions and Storage Time after Preparation of Syringes
- If Omacetaxine is not used immediately after reconstitution, follow in-use storage conditions and allowable storage times prior to use as instructed in Table 1. Do not administer Omacetaxine outside of the storage conditions and timeframes listed in TABLE 1.
Considerations for Home Administration
- Before a decision is made to allow Omacetaxine to be administered by someone other than a healthcare professional, ensure that the patient is an appropriate candidate for self-administration or for administration by a caregiver. Provide training on proper handling, storage conditions, administration, disposal, and clean-up of accidental spillage of the product. Ensure that patients receive the necessary supplies for home administration. At minimum these should include:
- Reconstituted Omacetaxine in syringe with a capped needle for subcutaneous injection. Syringe(s) should be filled to the patient-specific dose.
Protective eyewear
- Gloves
- An appropriate biohazard container
- Absorbent pad(s) for placement of administration materials and for accidental spillage
- Alcohol swabs
- Gauze pads
- Ice packs or cooler for transportation of reconstituted Omacetaxine syringes
- If a patient or caregiver cannot be trained for any reason, then in such patients, Omacetaxine should be administered by a healthcare professional.
Disposal and Accidental Spillage Procedures
- After administration, any unused solution should be discarded properly. Instruct patients planning home administration on the following: do not recap or clip the used needle, and do not place used needles, syringes, vials, and other used supplies in a household trash or recycling bin. Used needles, syringes, vials, and other used supplies should be disposed of in an appropriate biohazard container.
- If accidental spillage occurs, continue to use protective eyewear and gloves, wipe the spilled liquid with the absorbent pad, and wash the area with water and soap. Then, place the pad and gloves into the biohazard container and wash hands thoroughly. Return the biohazard container to the clinic or pharmacy for final disposal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Omacetaxine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Omacetaxine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Omacetaxine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Omacetaxine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Omacetaxine in pediatric patients.
Contraindications
- None.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Myelosuppression
- In uncontrolled trials with Omacetaxine , patients with chronic phase and accelerated phase CML experienced NCI CTC (version 3.0) Grade 3 or 4 thrombocytopenia (85%, 88%), neutropenia (81%, 71%), and anemia (62%, 80%), respectively. Fatalities related to myelosuppression occurred in 3% of patients in the safety population (N=163). Patients with neutropenia are at increased risk for infections, and should be monitored frequently and advised to contact a physician if they have symptoms of infection or fever.
- Monitor complete blood counts weekly during induction and initial maintenance cycles and every two weeks during later maintenance cycles, as clinically indicated. In clinical trials myelosuppression was generally reversible and usually managed by delaying next cycle and/or reducing days of treatment with Omacetaxine .
Bleeding
- Omacetaxine causes severe thrombocytopenia which increases the risk of hemorrhage. In clinical trials with CP and AP CML patients, a high incidence of Grade 3 and 4 thrombocytopenia (85% and 88%, respectively) was observed. Fatalities from cerebral hemorrhage occurred in 2% of patients treated with Omacetaxine in the safety population. Severe, non-fatal, gastrointestinal hemorrhages occurred in 2% of patients in the same population. Most bleeding events were associated with severe thrombocytopenia.
- Monitor platelet counts as part of the CBC monitoring as recommended . Avoid anticoagulants, aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs) when the platelet count is less than 50,000/µL as they may increase the risk of bleeding.
Hyperglycemia
- Omacetaxine can induce glucose intolerance. Grade 3 or 4 hyperglycemia was reported in 11% of patients in the safety population. Hyperosmolar non-ketotic hyperglycemia occurred in 1 patient treated with Omacetaxine in the safety population. Monitor blood glucose levels frequently, especially in patients with diabetes or risk factors for diabetes. Avoid Omacetaxine in patients with poorly controlled diabetes mellitus until good glycemic control has been established.
Embryo-Fetal Toxicity
- Omacetaxine can cause fetal harm when administered to a pregnant woman. Omacetaxine mepesuccinate caused embryo-fetal death in animals. Females of reproductive potential should avoid becoming pregnant while being treated with Omacetaxine . There are no adequate and well-controlled studies of Omacetaxine in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions have been associated with Omacetaxine in clinical trials and are discussed in greater detail in other sections of the label.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data for Omacetaxine are from 3 clinical trials which enrolled a total of 163 adult patients with TKI resistant and/or intolerant chronic phase (N=108) and accelerated phase (N=55) CML. All patients were treated with initial induction therapy consisting of a dose of 1.25 mg/m2 administered subcutaneously twice daily for 14 consecutive days every 28 days (induction cycle). Responding patients were then treated with the same dose and a twice daily schedule for 7 consecutive days every 28 days (maintenance cycle).
Clinical Trials Experience
Chronic Phase CML=
- The median duration of exposure for the 108 patients with chronic phase CML was 7.4 months (range 0 to 43 months). The median total cycles of exposure was 6 (range 1 to 41), and the median total dose delivered during the trials was 131 mg/m2 (range 1.2 to 678). Among the patients with chronic phase CML, 87% received 14 days of treatment during cycle 1. By cycles 2 and 3, the percentage of patients receiving 14 days of treatment decreased to 42% and 16% respectively. Of the 91 patients who received at least 2 cycles of treatment, 79 (87%) had at least 1 cycle delay during the trials. The median number of days of cycle delays was greatest for cycle 2 (17 days) and cycle 3 (25 days) when more patients were receiving induction cycles.
- Adverse reactions were reported for 99% of the patients with chronic phase CML. A total of 18% of patients had adverse reactions leading to withdrawal. The most frequently occurring adverse reactions leading to discontinuation were pancytopenia, thrombocytopenia, and increased alanine aminotransferase (each 2%). A total of 87% of patients reported at least 1 Grade 3 or Grade 4 treatment emergent adverse reaction (TABLE 2).There is limited information regarding Clinical Trial Experience of Omacetaxine in the drug label.
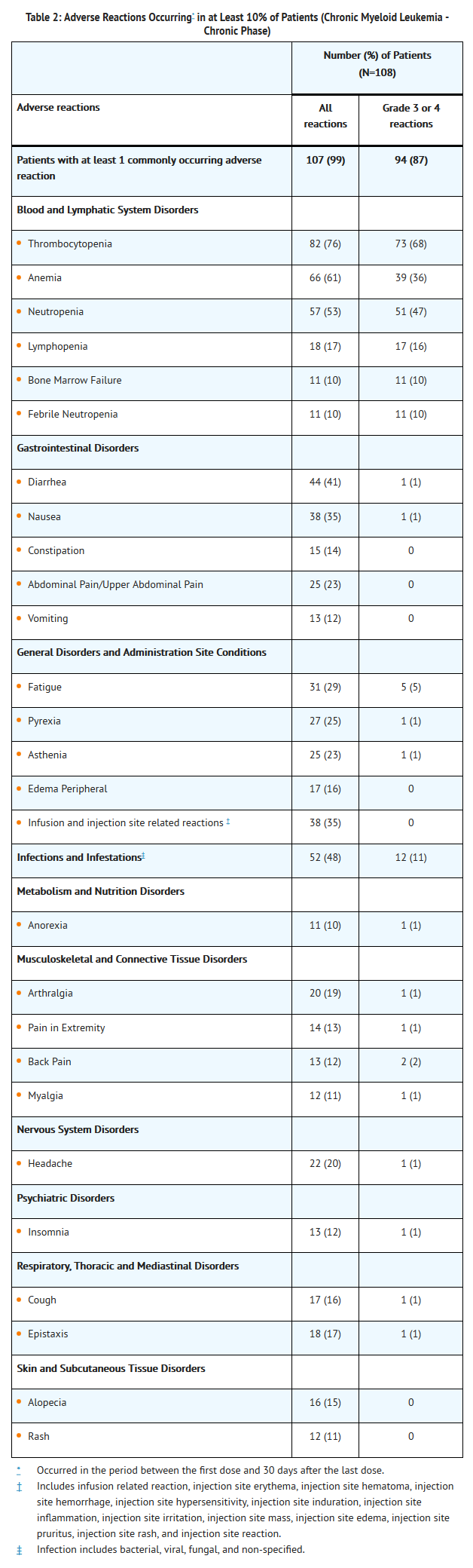
- Serious adverse reactions were reported for 51% of patients. Serious adverse reactions reported for at least 5% of patients were bone marrow failure and thrombocytopenia (each 10%), and febrile neutropenia (6%). Serious adverse reactions of infections were reported for 8% of patients.
- Deaths occurred while on study in five (5%) patients with CP CML. Two patients died due to cerebral hemorrhage, one due to multi-organ failure, one due to progression of disease, and one from unknown causes.
Accelerated Phase CML
- Median total cycles of exposure was 2 (range 1 to 29), and the median total dose delivered during the trials was 70 mg/m2. The median duration of exposure for the 55 patients with accelerated phase CML was 1.9 months (range 0 to 30 months). Of the patients with accelerated phase CML, 86% received 14 days of treatment during cycle 1. By cycles 2 and 3, the percentage of patients receiving 14 days of treatment decreased to 55% and 44% respectively. Of the 40 patients who received at least 2 cycles of treatment, 27 (68%) had at least 1 cycle delay during the trials. The median number of days of cycle delays was greatest for cycle 3 (31 days) and cycle 8 (36 days).
- Adverse reactions regardless of investigator attribution were reported for 100% patients with accelerated phase CML. A total of 33% of patients had adverse reactions leading to withdrawal. The most frequently occurring adverse reactions leading to withdrawal were leukocytosis (6%), and thrombocytopenia (4%). A total of 84% of patients reported at least 1 Grade 3 or Grade 4 treatment emergent adverse reaction.
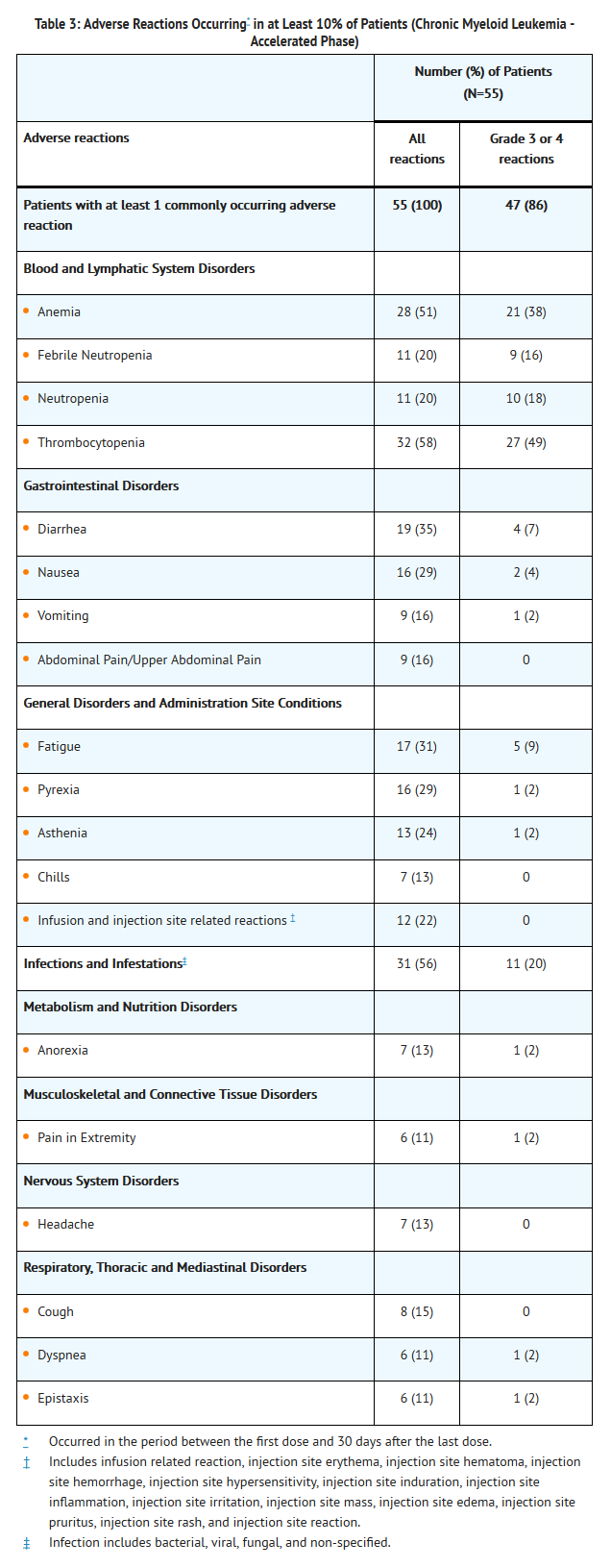
- Serious adverse reactions were reported for 60% of patients. Serious adverse reactions reported for at least 5% of patients were febrile neutropenia (18%), thrombocytopenia (9%), anemia (7%), and diarrhea (6%). Serious adverse reactions of infections were reported for 11% of patients.
- Death occurred while on study in 5 (9%) patients with AP CML. Two patients died due to cerebral hemorrhage and three due to progression of disease.
Laboratory Abnormalities in Chronic and Accelerated Phase CML
- Grade 3/4 laboratory abnormalities reported in patients with chronic and accelerated phase CML are described in TABLE 4. Myelosuppression occurred in all patients treated with Omacetaxine [see Warnings and Precautions (5.1)]. Five patients with chronic phase CML and 4 patients with accelerated phase CML permanently discontinued Omacetaxine due to pancytopenia, thrombocytopenia, febrile neutropenia, or bone marrow necrosis. An event of hyperosmolar non-ketotic hyperglycemia was reported in one patient in the safety population and a similar case has been reported in the literature. Two patients with chronic phase CML permanently discontinued Omacetaxine due to elevated transaminases.
Additional Data From Safety Population
- The following adverse reactions were reported in patients in the Omacetaxine clinical studies of patients with chronic phase and accelerated phase CML at a frequency of 1% to less than 10%. Within each category, adverse reactions are ranked on the basis of frequency.
- Cardiac Disorders: tachycardia, palpitations, acute coronary syndrome, angina pectoris, arrhythmia, bradycardia, ventricular extrasystoles.
- Ear and Labyrinth Disorders: ear pain, ear hemorrhage, tinnitus.
- Eye Disorders: cataract, vision blurred, conjunctival hemorrhage, dry eye, lacrimation increased, conjunctivitis, diplopia, eye pain, eyelid edema.
- Gastrointestinal Disorders: stomatitis, mouth ulceration, abdominal distension, dyspepsia, gastroesophageal reflux disease, gingival bleeding, aphthous stomatitis, dry mouth, hemorrhoids, gastritis, gastrointestinal hemorrhage, melena, mouth hemorrhage, oral pain, anal fissure, dysphagia, gingival pain, gingivitis.
- General Disorders and Administration Site Conditions: mucosal inflammation, pain, chest pain, hyperthermia, influenza-like illness, catheter site pain, general edema, malaise.
- Immune System Disorders: hypersensitivity.
- Injury, Poisoning and Procedural Complications: contusion, transfusion reaction.
- Metabolism and Nutrition Disorders: decreased appetite, diabetes mellitus, gout, dehydration.
- Musculoskeletal and Connective Tissue Disorders: bone pain, myalgia, muscular weakness, muscle spasms, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal stiffness, musculoskeletal discomfort.
- Nervous System Disorders: dizziness, cerebral hemorrhage, paresthesia, convulsion, hypoesthesia, lethargy, sciatica, burning sensation, dysgeusia, tremor.
- Psychiatric Disorders: anxiety, depression, agitation, confusional state, mental status change.
- Renal and Urinary Disorders: dysuria.
- Respiratory, Thoracic and Mediastinal Disorders: pharyngolaryngeal pain, nasal congestion, dysphonia, productive cough, rales, rhinorrhea, hemoptysis, sinus congestion.
- Skin and Subcutaneous Tissue Disorders: erythema, pruritus, dry skin, petechiae, hyperhidrosis, night sweats, ecchymosis, purpura, skin lesion, skin ulcer, rash erythematous, rash papular, skin exfoliation, skin hyperpigmentation.
- Vascular Disorders: hematoma, hypertension, hot flush, hypotension.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Omacetaxine in the drug label.
Drug Interactions
- Based on the findings from in vitro drug interaction studies with Omacetaxine , no clinical drug interaction trials were warranted
Use in Specific Populations
Pregnancy
- Pregnancy Category D
Risk Summary
- Based on its mechanism of action and findings from animal studies, Omacetaxine can cause fetal harm when administered to pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Animal Data
- In an embryo-fetal development study, pregnant mice were administered omacetaxine mepesuccinate subcutaneously during the period of organogenesis at doses of 0.21 or 0.41 mg/kg/day. Drug-related adverse effects included embryonic death, an increase in unossified bones/reduced bone ossification and decreased fetal body weights. Fetal toxicity occurred at doses of 0.41 mg/kg (1.23 mg/m2) which is approximately half the recommended daily human dose on a body surface area basis.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Omacetaxine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Omacetaxine during labor and delivery.
Nursing Mothers
- It is not known whether omacetaxine mepesuccinate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reaction in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Omacetaxine in pediatric patients have not been established
Geriatic Use
- In the chronic and accelerated phase CML efficacy populations 23 (30%) and 16 (46%) patients were ≥65 years of age. For the age subgroups of <65 years of age and ≥65 years of age, there were differences between the subgroups, with higher rates of major cytogenetic responses (MCyRs) in younger patients with CP CML compared with older patients (23% vs. 9%, respectively) and higher rates of major hematologic responses (MaHRs) in older patients with AP CML compared with younger patients (31% vs. 0%, respectively). Patients ≥65 years of age were more likely to experience toxicity, most notably hematologic toxicity.
Gender
- Of the 76 patients included in the chronic phase CML population efficacy analysis, 47 (62%) of the patients were men and 29 (38%) were women. For patients with chronic phase CML, the MCyR rate in men was higher than in women (21% vs. 14%, respectively). There were differences noted in the safety profile of omacetaxine mepesuccinate in men and women with chronic phase CML although the small number of patients in each group prevents a definitive assessment. There were inadequate patient numbers in the accelerated phase subset to draw conclusions regarding a gender effect on efficacy.
Race
There is no FDA guidance on the use of Omacetaxine with respect to specific racial populations.
Renal Impairment
- No formal studies assessing the impact of renal impairment on the pharmacokinetics of omacetaxine mepesuccinate have been conducted.
Hepatic Impairment
- No formal studies assessing the impact of hepatic impairment on the pharmacokinetics of omacetaxine mepesuccinate have been conducted.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Omacetaxine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Omacetaxine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Omacetaxine in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Omacetaxine in the drug label.
Overdosage
- A patient in the clinical expanded access program received an overdose of 2.5 mg/m2 twice daily for 5 days in the 16th cycle. The patient presented with gastrointestinal disorders, gingival hemorrhage, alopecia, and Grade 4 thrombocytopenia and neutropenia. When Omacetaxine treatment was temporarily interrupted the gastrointestinal disorders and hemorrhagic syndrome resolved, and neutrophil values returned to within normal range. The alopecia and thrombocytopenia (Grade 1) improved, and Omacetaxine was restarted.
- No specific antidote for Omacetaxine overdose is known. Management of overdosage should include general supportive measures, including monitoring of hematologic parameters.
There is limited information regarding Chronic Overdose of Omacetaxine in the drug label.
Pharmacology
Mechanism of Action
- The mechanism of action of omacetaxine mepesuccinate has not been fully elucidated but includes inhibition of protein synthesis and is independent of direct Bcr-Abl binding. Omacetaxine mepesuccinate binds to the A-site cleft in the peptidyl-transferase center of the large ribosomal subunit from a strain of archaeabacteria. In vitro, omacetaxine mepesuccinate reduced protein levels of the Bcr-Abl oncoprotein and Mcl-1, an anti-apoptotic Bcl-2 family member. Omacetaxine mepesuccinate showed activity in mouse models of wild-type and T315I mutated Bcr-Abl CML.
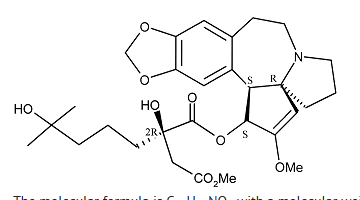
Structure
- Omacetaxine contains the active ingredient omacetaxine mepesuccinate, a cephalotaxine ester. It is a protein synthesis inhibitor. Omacetaxine mepesuccinate is prepared by a semi-synthetic process from cephalotaxine, an extract from the leaves of Cephalotaxus sp. The chemical name of omacetaxine mepesuccinate is cephalotaxine, 4-methyl (2R)-hydroxyl-2-(4-hydroxyl-4-methylpentyl) butanedioate (ester).
Omacetaxine mepesuccinate has the following chemical structure:
- The molecular formula is C29H39NO9 with a molecular weight of 545.6 g/mol. Omacetaxine for Injection is a sterile, preservative-free, white to off-white, lyophilized powder in a single-use vial. Each vial contains 3.5 mg omacetaxine mepesuccinate and mannitol.
- Omacetaxine is intended for subcutaneous administration after reconstitution with 1.0 mL of 0.9% Sodium Chloride Injection, USP. The pH of the reconstituted solution is between 5.5 and 7.0.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Omacetaxine in the drug label.
Pharmacokinetics
- The dose proportionality of omacetaxine mepesuccinate is unknown. A 90% increase in systemic exposure to omacetaxine mepesuccinate was observed between the first dose and steady state.
Absorption
- The absolute bioavailability of omacetaxine mepesuccinate has not been determined. Omacetaxine mepesuccinate is absorbed following subcutaneous administration, and maximum concentrations are achieved after approximately 30 minutes.
Distribution
- The steady-state (mean ± SD) volume of distribution of omacetaxine mepesuccinate is approximately 141 ± 93.4 L following subcutaneous administration of 1.25 mg/m2 twice daily for 11 days . *The plasma protein binding of omacetaxine mepesuccinate is less than or equal to 50%.
Metabolism
- Omacetaxine mepesuccinate is primarily hydrolyzed to 4′-DMHHT via plasma esterases with little hepatic microsomal oxidative and/or esterase-mediated metabolism in vitro.
Elimination
- The major elimination route of omacetaxine mepesuccinate is unknown. The mean percentage of omacetaxine mepesuccinate excreted unchanged in the urine is less than 15%. The mean half-life of omacetaxine mepesuccinate following subcutaneous administration is approximately 6 hours.
Drug Interactions
- Cytochrome P450 Enzymes (CYPs): Omacetaxine mepesuccinate is not a substrate of CYP450 enzymes in vitro. Omacetaxine mepesuccinate and 4′-DMHHT do not inhibit major CYPs in vitro at concentrations that can be expected clinically. The potential for omacetaxine mepesuccinate or 4′-DMHHT to induce CYP450 enzymes has not been determined.
- Transporter Systems: Omacetaxine mepesuccinate is a P-glycoprotein (P-gp) substrate in vitro. Omacetaxine mepesuccinate and 4′-DMHHT do not inhibit P-gp mediated efflux of loperamide in vitro at concentrations that can be expected clinically.
Assessment for Risk of QT Prolongation
- In an uncontrolled pharmacokinetic study there were no reports of QTcF > 480 ms or ΔQTcF > 60 ms in 21 treated patients who received omacetaxine mepesuccinate 1.25 mg/m2 BID for 14 consecutive days. There was no evidence for concentration-dependent increases in QTc for omacetaxine mepesuccinate or 4’-DMHHT. Although the mean effect on QTc was 4.2 ms (upper 95% CI: 9.5 ms), QTc effects less than 10 ms cannot be verified due to the absence of a placebo and positive controls.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity studies have been conducted with omacetaxine mepesuccinate.
- Omacetaxine mepesuccinate was genotoxic in an in vitro chromosomal aberration test system in Chinese hamster ovary (CHO) cells, but was not mutagenic when tested in an in vitro bacterial cell assay (Ames test), and it did not induce genetic damage using an in vivo mouse micronucleus assay.
- Omacetaxine may impair male fertility. Studies in mice demonstrated adverse effects on male reproductive organs. Bilateral degeneration of the seminiferous tubular epithelium in testes and hypospermia/aspermia in the epididymides were reported in the highest dose group (2.33 mg/kg/day reduced to 1.67 mg/kg/day; 7 to 5 mg/m2/day) following subcutaneous injection of omacetaxine mepesuccinate for six cycles over six months. The doses used in the mice were approximately two to three times the clinical dose (2.5 mg/m2/day) based on body surface area.
Clinical Studies
- The efficacy of Omacetaxine was evaluated using a combined cohort of adult patients with CML from two trials. The combined cohort consisted of patients who had received 2 or more approved TKIs and had, at a minimum, documented evidence of resistance or intolerance to dasatinib and/or nilotinib. Resistance was defined as one of the following: no complete hematologic response (CHR) by 12 weeks (whether lost or never achieved); or no cytogenetic response by 24 weeks (i.e., 100% Ph positive [Ph+]) (whether lost or never achieved); or no major cytogenetic response (MCyR) by 52 weeks (i.e., ≥35% Ph+) (whether lost or never achieved); or progressive leukocytosis. Intolerance was defined as one of the following: 1) Grade 3-4 non-hematologic toxicity that does not resolve with adequate intervention; or 2) Grade 4 hematologic toxicity lasting more than 7 days; or 3) any Grade ≥ 2 toxicity that is unacceptable to the patient. Patients with NYHA class III or IV heart disease, active ischemia or other uncontrolled cardiac conditions were excluded.
- Patients were treated with omacetaxine mepesuccinate at a dose of 1.25 mg/m2 administered subcutaneously twice daily for 14 consecutive days every 28 days (induction cycle). Responding patients were then treated with the same dose and twice daily schedule for 7 consecutive days every 28 days (maintenance cycle). Patients were allowed to continue to receive maintenance treatment for up to 24 months. Responses were adjudicated by an independent Data Monitoring Committee (DMC).
Chronic Phase CML (CP CML)
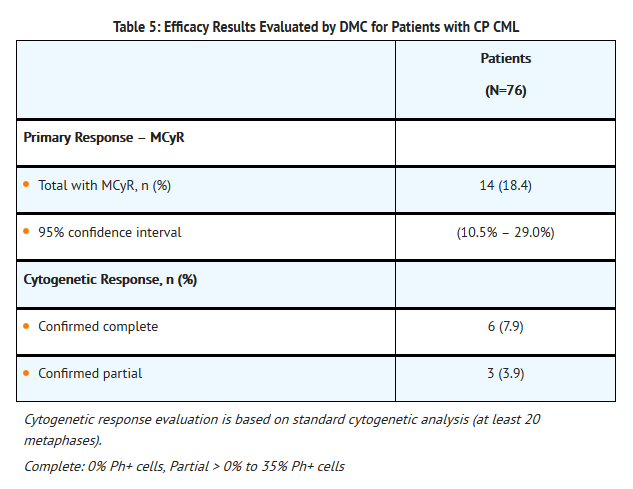
- A total of 76 patients with chronic phase CML were included in the efficacy analysis. The demographics were: median age 59 years, 62% were male, 30% were 65 years of age or older, 80% were Caucasian, 5% were African-American, 4% were Asian and 4% were Hispanic. Thirty-six (47%) patients had failed treatment with imatinib, dasatinib, and nilotinib. Most patients had also received prior non-TKI treatments, most commonly hydroxyurea (54%), interferon (30%), and/or cytarabine (29%). The efficacy endpoint was based on MCyR (adjudicated by a DMC).

- The mean time to MCyR onset in the 14 patients was 3.5 months. The median duration of MCyR for the 14 patients was 12.5 months (Kaplan-Meier estimate).
Accelerated Phase CML (AP CML)
- A total of 35 patients with accelerated phase CML were included in the efficacy analysis. The demographics were: median age was 63 years, 57% were male, 46% were 65 years of age or older, 68% were Caucasian, 23% were African-American, 3% were Asian and 3% were Hispanic. Twenty-two (63%) of 35 patients with accelerated phase had failed treatment with imatinib, dasatinib, and nilotinib. Most patients had also received prior non-TKI treatments, most commonly hydroxyurea (43%), interferon (31%), and/or cytarabine (29%). The efficacy endpoint was assessed based on MCyR and MaHR (complete hematologic response [CHR] or no evidence of leukemia [NEL]). The efficacy results for the patients with accelerated phase as adjudicated by the DMC
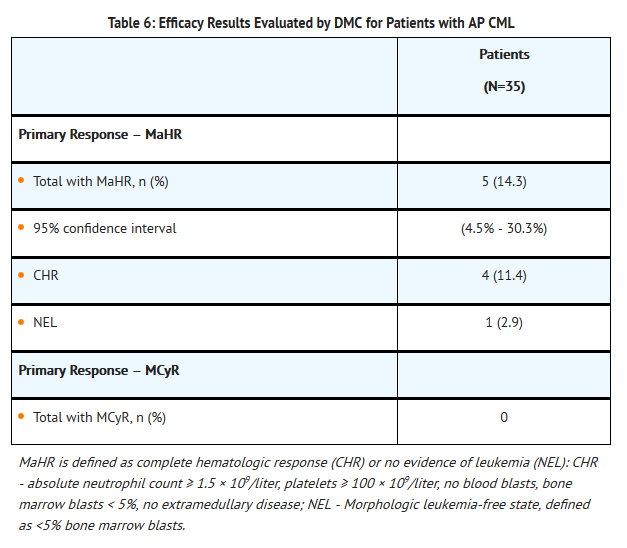
- The mean time to response onset in the 5 patients was 2.3 months. The median duration of MaHR for the 5 patients was 4.7 months (Kaplan-Meier estimate).
How Supplied
- Omacetaxine (omacetaxine mepesuccinate) for Injection is supplied in 8 mL clear glass single-use vial in individual cartons. Each vial contains 3.5 mg of Omacetaxine (omacetaxine mepesuccinate) for Injection (NDC 63459-177-14).
Storage and Handling
- Store unopened vials at 20oC to 25ºC (68o F to 77ºF); excursions permitted from 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Prior to re-constitution, keep product in carton to protect from light.Omacetaxine mepesuccinate is a cytotoxic drug. Follow special handling and disposal procedures1.
Storage
There is limited information regarding Omacetaxine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Omacetaxine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Omacetaxine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Assist patients and caregivers in understanding their contents and give them the opportunity to discuss the contents of the Medication Guide and Instructions for Use and to obtain answers to any questions they may have prior to initiating therapy. The complete text of the Medication Guide and Instructions for Use are attached to the prescribing information.
Patient Training
- Once it is determined that a patient is an appropriate candidate for self-administration or administration by a caregiver, ensure that patients receive the necessary supplies for home administration of Omacetaxine and train them on the following.
- How to transport reconstituted Omacetaxine in a secure container or packaging and under recommended temperature conditions
- Acceptable storage conditions and use times for reconstituted Omacetaxine
- When stored in a refrigerator (2°C to 8°C [36°F to 46°F]), use within 6 days (144 hours)
- When stored at room temperature (not to exceed 25°C [77°F]), use within 12 hours
- If stored in a refrigerator, keep Omacetaxine from coming into contact with food or drink.
- To wear disposable gloves and protective eyewear when handling Omacetaxine .
- To wash hands before putting on gloves and after removing gloves.
- Not to eat or drink while handling Omacetaxine . To administer Omacetaxine in an area away from food or food preparation areas.
- To administer Omacetaxine in a location away from children and pregnant women.
- Proper subcutaneous injection technique including acceptable sites.
- The importance of body site selection for administering the injection, as well as the importance of alternating the injection sites. Advise patients to not inject Omacetaxine into areas of the skin that are tender, red, bruised, hard, or that have scars or stretch marks.
- In the case of a missed dose: If a patient misses an injection, skip the missed dose and the patient should give the next scheduled injection at the next scheduled time. Inform patients NOT to give two injections to make up for a missed injection.
- In the case that Omacetaxine comes into contact with a patient’s skin or eyes: Advise patients to wash exposed skin with soap and water and in the case of eye exposure, thoroughly flush the eye with water. After washing or flushing, advise patients to call their healthcare provider immediately.
- In the case that too much Omacetaxine is injected or that Omacetaxine is accidentally swallowed: Instruct patients to contact their healthcare provider immediately if they have injected too much Omacetaxine , or if someone has swallowed Omacetaxine .
- Disposal procedures, including use of an appropriate biohazard container and return of the container to the clinic or pharmacy for final disposal. Inform patients NOT to recap or clip the used needle and not to place used needles, syringes, vials, and other used supplies in a household trash or recycle container.
- Accidental spillage procedures, including wiping the spilled liquid with the absorbent pad (using protective eyewear and gloves), washing the area with water and soap, and proper disposal of materials.
Bleeding
- Advise patients of the possibility of serious bleeding due to low platelet counts. Instruct patients to report immediately any signs or symptoms suggestive of hemorrhage (unusual bleeding, easy bruising or blood in urine or stool; confusion, slurred speech, or altered vision). Instruct patients to report in advance if they plan to have any dental or surgical procedures.
Myelosuppression
- Advise patients of the likelihood that Omacetaxine will cause a decrease in white blood cells, platelets, and red blood cells and that monitoring of these parameters will be needed. Instruct patients to contact a health care professional if they develop a fever, or other signs/symptoms of infection; shortness of breath, significant fatigue, or bleeding.
Hyperglycemia
- Advise patients with diabetes of the possibility of hyperglycemia and the need for careful monitoring of blood glucose levels. Patients with poorly controlled diabetes mellitus should not be treated with omacetaxine mepesuccinate until good glycemic control has been established.
Pregnancy and Nursing
- Advise patients that omacetaxine mepesuccinate can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential of the potential hazard to the fetus and to avoid becoming pregnant. Advise females to avoid nursing while receiving Omacetaxine .
Gastrointestinal Distress
- Advise patients that they may experience nausea, diarrhea, abdominal pain, constipation, and vomiting. If these symptoms persist, they should seek medical attention.
Fatigue
- Advise patients that Omacetaxine may cause tiredness and to avoid driving any vehicle or operating any dangerous tools or machinery if they experience this side effect.
Rash
- Advise patients that they may experience skin rash. Advise patients to immediately report severe or worsening rash or itching.
Alopecia
- Advise patients that they may experience hair loss.
Precautions with Alcohol
- Alcohol-Omacetaxine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Omacetaxine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Omacetaxine |Label Name=Omacetaxine07.png
}}
{{#subobject:
|Label Page=Omacetaxine |Label Name=Omacetaxine08.png
}}
{{#subobject:
|Label Page=Omacetaxine |Label Name=Omacetaxine 09.png
}}